You are here: Urology Textbook > Kidneys > Renal cell carcinoma > Surgical Treatment
Surgical Treatment for Renal Cell Carcinoma
- Renal cell carcinoma: Definition and Epidemiology
- Renal cell carcinoma: Pathology
- Renal cell carcinoma: Diagnostic workup
- Renal cell carcinoma: Surgical Treatment
- Renal cell carcinoma: Targeted therapy of advanced disease
Overview of Treatment Options for Renal Cell Carcinoma
Unilateral small kidney tumor:
Open partial nephrectomy or laparoscopic partial nephrectomy is the standard treatment, depending on the surgeon's technical expertise. Good-located tumors can be treated using radiofrequency ablation or cryotherapy. Active monitoring is possible in elderly patients with significant comorbidity.
Large unilateral kidney tumor:
Radical nephrectomy is the standard treatment for large tumors infiltrating the renal hilum or with a venous thrombus. Depending on the stage of the disease and the surgeon's technical expertise, radical nephrectomy can be performed laparoscopically, retroperitoneoscopically, via flank incision, or with a transperitoneal approach.
Bilateral kidney tumors:
Partial nephrectomy should be strived for on both sides. If this seems impossible, a partial nephrectomy is performed on the technically simpler side. This avoids requiring dialysis after temporary ischemia (with the help of the contralateral kidney). After one month, the remaining kidney function (scintigraphy) is evaluated. If the renal function after partial nephrectomy is sufficient, radical nephrectomy can be performed on the contralateral side. If the renal function is insufficient, an imperative partial nephrectomy (using all technical possibilities) of the contralateral side should be tried, alternatively radical nephrectomy with subsequent dialysis.
Metastatic renal cell carcinoma:
Surgery is not indicated for patients with a poor prognosis. Surgical treatment options have priority before starting systemic therapy in patients with favorable prognosis if they can significantly reduce the tumor burden and if the patient is in good general condition:
- cytoreductive tumor nephrectomy
- lymphadenectomy of enlarged lymph nodes
- resection of one or more metastases
If all tumor manifestations could be removed with surgery, there is no indication for adjuvant systemic therapy. Multiple metastases or single metastasis that cannot be removed surgically are treated systemically with inhibitors of signal transduction or checkpoint inhibitors.
Radical Nephrectomy for Renal Cell Carcinoma
Indication For Radical Nephrectomy:
Radical nephrectomy is the standard of care for a large renal cell carcinoma or RCC with a cava thrombus.
Relative Contraindications for Nephrectomy:
Partial nephrectomy should be the preferred treatment independent of size if technically possible. Organ-sparing is especially important for patients with impending renal failure: increased serum creatinine, proteinuria, diabetes mellitus, arterial hypertension, bilateral renal cell carcinomas, or familial renal cell carcinoma.
Surgical technique of radical nephrectomy:
Various surgical techniques are possible: laparoscopic (robot-assisted) nephrectomy, retroperitoneoscopic nephrectomy, open transperitoneal nephrectomy, thoraco-abdominal nephrectomy, or nephrectomy via a flank incision (Robson et al., 2002).
Laparoscopic and retroperitoneoscopic surgery has lower morbidity and is oncologically equivalent (Hemal et al., 2007). There are no significant clinical differences between the laparoscopic or retroperitoneoscopic approach (Desai et al., 2005). Laparoscopic nephrectomy has become the method of choice for T1–2 renal tumors, which are unsuitable for partial nephrectomy and if the tumor size allows the endoscopic technique (EAU guideline, Ljungberg et al., 2010).
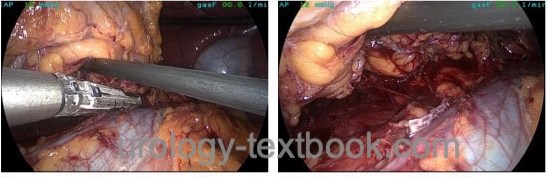 |
Open nephrectomy via a midline incision, subcostal incision, or flank incision is preferred for an advanced local tumor stage, e.g., vein invasion with tumor thrombus, infiltration of neighboring organs, lymph node enlargement, or large tumor diameter. Open surgery is also advisable if significant intraabdominal adhesions from previous surgery are suspected or if the surgeon's technical skills are inadequate for laparoscopy.
Treatment of renal cell carcinoma with venous tumor thrombus:
Important for treatment planning is the accurate determination of the cranial extension of the tumor thrombus to enable clamping of the vena cava:
- Level I: tumor thrombus in the renal vein
- Level II: tumor thrombus extending into the infrahepatic vena cava
- Level III: tumor thrombus extending into the intrahepatic vena cava below the diaphragm
- Level IV: tumor thrombus extending above the diaphragm
For technical details of the surgical management, please see the section radical nephrectomy.
Adrenalectomy:
The need for routine adrenalectomy is hardly seen today. The adrenal gland is separated from the renal adipose capsule and spared using blunt and sharp dissection. The involvement of the adrenal gland (1-3%) rarely expresses continuous tumor growth but often represents metastasis with a poor prognosis (Han et al., 2003). The probability of correct imaging of adrenal involvement using CT is 97%. If adrenal involvement is suspected, adrenalectomy is performed together with radical nephrectomy.
Regional lymphadenectomy:
Lymphadenectomy is unnecessary for T1–2 tumors without suspicious lymph node enlargement, since no survival benefit could be demonstrated in a large EORTC study (Blom et al., 1999 and 2009). Some authors advocate an extended lymphadenectomy if an advanced tumor or enlarged lymph nodes are present. Extended lymphadenectomy is done from the crus of the diaphragm to the aortic bifurcation. For left-sided tumors, the para-aortic lymph nodes are removed. The paracaval lymph nodes are removed for right-sided tumors, and the interaortocaval lymph nodes should be removed for either side (Capitanio et al., 2011).
Oncological results after nephrectomy:
See table Robson stage and survival for the tumor-specific survival rates depending on the clinical stage. More precise results for the survival probability are possible by considering several risk factors, see stage, size, grade, and necrosis (SSIGN) Score [table Assessing SSIGN Score and Survival and SSIGN Score].
| Clinical tumor stage | Five-year survival rate |
| Stadium I (limited to the kidney) | 75–92 % |
| Stadium II (limited within the renal fascia) | 63–77 % |
| Stadium III (venous invasion, pN+) | 38–47 % |
| Stadium IV (pT4 or M1) | 11–12 % |
| Risk factor | Score |
| T stage | |
| T1 | 0 |
| T2 | 1 |
| T3 | 2 |
| T4 | 4 |
| Lymph node stage | |
| pNx oder pN0 | 0 |
| pN1 oder pN2 | 2 |
| Metastasis | |
| M0 | 0 |
| M1 | 4 |
| Tumor size | |
| <5 cm | 0 |
| >5 cm | 2 |
| Grading | |
| Grade 1–2 | 0 |
| Grade 3 | 1 |
| Grade 4 | 3 |
| Tumor necrosis | |
| Not present | 0 |
| present | 2 |
| SSIGN-Score | 1 year | 5 years | 10 years |
| 0–2 | 99% | 97% | 94% |
| 3–4 | 98% | 90% | 78% |
| 5–6 | 93% | 74% | 57% |
| 7–9 | 77% | 39% | 26% |
| >10 | 43% | 19% | 19% |
Adjuvant therapy after nephrectomy:
There is a high risk of disease progression for advanced renal cell carcinoma (pN+, T4, or G3–4 tumors). The therapeutic options for metastatic renal cell carcinoma are limited. Tumor recurrence depends, among other things, on the function of the immune system and thus can theoretically be influenced by adjuvant therapy.
Adjuvant therapy with checkpoint inhibitors:
Keynote-564 is the first phase III trial to demonstrate improved disease-free survival (HR 0.72) and an improved overall survival (91% vs. 86%) due to adjuvant therapy with pembrolizumab after surgical therapy (Choureiri u.a., 2024). Patients with dedifferentiated tumors (grade 4), T3–4, N+, or after complete metastatic resection were included. The approval was granted 11/2021 by the FDA and 2/2022 by the EMA. There are clear disadvantages of early adjuvant therapy with pembrolizumab: overtreatment of non-metastatic patients, undertreatment of metastatic patients (no TKI), and high costs of more than 100000 Euro.
Adjuvant therapy with tyrosine kinase inhibitors:
Several randomized studies examined adjuvant therapy with signal transduction inhibitors in patients at high risk of progression after surgery: ASSURE (sunitinib vs. sorafenib vs. placebo) showed no significant advantages (Haas et al., 2017). PROTECT (pazopanib vs. placebo) improved progression-free survival (HR 0.69), but no difference in overall survival could be demonstrated (Motzer et al., 2017). S-TRAC (sunitinib vs. placebo) was able to demonstrate an improvement in progression-free survival (97 vs. 122 of 615 patients developed metastases), overall survival has not benn published yet (Motzer et al., 2018). There are significant side effects with long-term administration of TKI, adjuvant therapy is currently not recommended without inclusion into controlled trials, and available signal transduction inhibitors are not approved for adjuvant therapy (Sun et al., 2018).
Follow-up after radical nephrectomy:
There are no firm recommendations for follow-up of renal cell carcinoma. According to the recommendations of the EAU and the German S3 guideline, follow-up examinations should be done depending on the symptoms and the likelihood of metastasis. The probability of metastasis can be assessed using the Stage, Size, Grade, and Necrosis (SSIGN) score (Assessing SSIGN Score and Survival and SSIGN Score).
For low-risk patients, follow-up is recommended for five years. Clinical examinations, laboratory, and ultrasound controls are recommended in months 3, 6, 12, 24, 36, 48, and 60. The EAU guideline recommends a CT examination only for symptoms typical for recurrence. The German S3 guideline recommends a CT of the chest and abdomen after 1, 2, and 4 years.
For patients with moderate and high risk, the recommended duration of follow-up is nine years. Clinical examinations, laboratory, and ultrasound controls are recommended in months 3, 6, 12, 24, 36, 48, 60, 84, and 108. CT of chest and abdomen after 1, 2, 3, 4, 5, 7, and 9 years. For high-risk patients, perform additional chest CTs in months 6 and 18. Bone scintigraphy is indicated for patients with bone pain.
Local recurrence after surgical therapy of kidney cancer:
Local recurrence without evidence of distant metastases should be surgically removed whenever possible. If complete resection is possible, the five-year survival rate is around 50%.
Imperative indications for partial nephrectomy:
Partial nephrectomy is imperative if radical nephrectomy results in renal insufficiency requiring dialysis. If oncologically possible, partial nephrectomy is strongly indicated for:
- RCC in a single kidney
- Chronic kidney disease
- Bilateral tumors
- Familial renal tumors
- Known diseases with imminent future renal insufficiency (e.g., diabetes mellitus or renal artery stenosis).
Elective indications for partial nephrectomy:
Current guidelines prefer partial nephrectomy over radical nephrectomy. If technically possible, organ-preserving partial nephrectomy should be performed for any T1 tumor. Retrospective comparisons between nephrectomy and partial nephrectomy for T1 tumors showed a better survival rate of patients after partial nephrectomy. This is explained with a reduction of cardiovascular disease due to better renal function (Zini et al., 2009) (Weight et al., 2010) (Sun et al., 2012). The randomized EORTC study (nephrectomy vs. partial nephrectomy) could not demonstrate this effect (van Poppel et al., 2011).
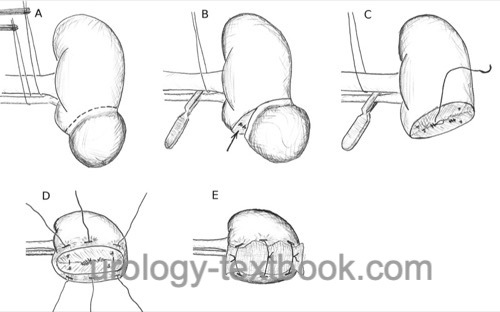
Surgical technique of partial nephrectomy:
Open partial nephrectomy is done via a lumbar or, less often, via a transperitoneal access to the kidney. Laparoscopic (robotic-assisted) partial nephrectomy is possible with a transperitoneal or retroperitoneoscopic approach. Vascular control is mandatory. Tumor excision is accomplished with or without the use of temporary ischemia, depending on tumor size and location. The safety margin to the tumor should be 5–10 mm, negative surgical margins are important. For technical details please refer to section open partial nephrectomy.
 |
Laparoscopic (robot-assisted) techniques offer advantages in postoperative pain and reduce complications related to the surgical approach. Oncologic outcomes and blood loss are comparable. Evidence regarding intraoperative warm ischemia time is mixed. Robotic-assisted laparoscopy is a promising approach that simplifies laparoscopy and enables complex partial nephrectomies for centrally located renal tumors (Xia et al., 2017).
Approach to bilateral renal cell carcinoma:
The simpler partial nephrectomy is done first. This avoids requiring dialysis due to transient ischemia of a single kidney. After full recovery from surgery, the residual renal function is determined with renal scintigraphy. With sufficient residual renal function, complex or central contralateral tumors may be treated with radical nephrectomy. In case of insufficient residual renal function, imperative partial nephrectomy should be attempted, if technically possible.
Oncological results after partial nephrectomy:
The randomized EORTC study (radical nephrectomy vs. partial nephrectomy, n = 541) proofed the oncological safety of partial nephrectomy (van Poppel et al., 2011): only 12 of the 117 deaths were caused by advanced renal cell carcinoma (4 vs. 8). 21 patients experienced tumor progression (9 vs. 12). The oncological results are depending (comparable to radical nephrectomy) on the tumor stage, see Table oncological results after partial nephrectomy. The local recurrence after partial nephrectomy occurs between 1.4–10% and depends on patient selection and tumor size. Survival after partial nephrectomy depends on the appearance of distant metastases, which most often arise without local recurrence.
Significance of Positive Surgical Margins:
Partial nephrectomy can be done without frozen section examination if an intraoperative macroscopic R0 resection can be achieved. The risk of a microscopic R1 resection is then 0–7% depending on the tumor size and grading. However, the tumor-specific survival is not influenced by the presence of the R1 finding; the most critical variables remain tumor size and grading. A prophylactic secondary nephrectomy in the case of a microscopic R1 finding is not recommended, tumor follow-up care and nephrectomy in case of local recurrence are sufficient.
Follow-up after partial nephrectomy:
After R1 resection or minimal safety margin, perform an abdominal CT scan three months after surgery to document postoperative alterations. Further follow-up after partial nephrectomy should be done depending on symptoms and the likelihood of metastasis. The probability of metastasis can be assessed using the Stage, Size, Grade, and Necrosis (SSIGN) score (Assessing SSIGN Score and Survival and SSIGN Score).
Experimental therapy for localized renal cell carcinoma
Cryotherapy, radiofrequency ablation, or active surveillance are options for selected patients. Biopsy of the renal mass to ensure malignancy is recommended before invasive treatment (with complications up to 20%). All options lack long-term results.
Cryotherapy of renal cell carcinoma:
Repetitive freezing and thawing are applied to the tumor tissue via a percutaneous or laparoscopic technique. Reliable cell destruction requires several freeze-thaw cycles with rapid freezing and gradual thawing. The frozen tissue is replaced by granulation tissue. There are several advantages to laparoscopic cryotherapy: the mobilized kidney can be cooled better, the risk of injury to adjacent organs is minimized, and there is a visual control of the "ice ball" with respect to the tumor margin.
Radiofrequency ablation of renal cell carcinoma:
Peripher tumors up to 5 cm can be treated with radiofrequency ablation. Compared to cryotherapy, visual control of the treatment effect is more demanding, and experience in this new technique is limited.
Active surveillance for T1a renal cell carcinoma:
In case of severe comorbidity and advanced age, active surveillance is a treatment option for small renal tumors. If the tumor diameter less than 3 cm, 22–46% of renal tumors are benign, and only 10% of renal cell carcinomas are poorly differentiated [Table tumor pathology in relation of tumor size]. Active surveillance should be reconsidered if significant growth of the tumor is detected or if the tumor diameter exceeds 3–4 cm.
| Tumor size [cm] | Benign tumors [%] | Malignant tumors [%] | High-grade RCC [%] |
| 0 to 0.9 | 46 | 54 | 9 |
| 1 to 1.9 | 22 | 78 | 11 |
| 2 to 2.9 | 22 | 78 | 7 |
| 3 to 3.9 | 20 | 80 | 19 |
| 4 to 4.9 | 10 | 90 | 22 |
| 5 to 5.9 | 13 | 87 | 31 |
| 6 to 6.9 | 5 | 95 | 39 |
| >7 | 6 | 94 | 62 |
| RCC diagnosis | Index | RCC metastasis therapy |
Index: 1–9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
References
Blom u.a. 1999 BLOM, J. H. ; POPPEL, H. van ;
MARECHAL, J. M. ; JACQMIN, D. ; SYLVESTER, R. ;
SCHRODER, F. H. ; PRIJCK, L. de:
Radical nephrectomy with and without lymph node dissection:
preliminary results of the EORTC randomized phase III protocol 30881. EORTC
Genitourinary Group.
In: Eur Urol
36 (1999), Nr. 6, S. 570–5
Blom, Jan H M; van Poppel, Hein; Maréchal, Jean M;
Jacqmin, Didier; Schröder, Fritz H; de Prijck, Linda; Sylvester, Richard &
E. O. R. T. C. Genitourinary Tract Cancer Group
Radical nephrectomy
with and without lymph-node dissection: final results of European
Organization for Research and Treatment of Cancer (EORTC) randomized phase
3 trial 30881.
Eur Urol, 2009, 55, 28-34.
Capitanio, U.; Becker, F.; Blute, M. L.; Mulders, P.;
Patard, J.; Russo, P.; Studer, U. E. & Poppel, H. V.
Lymph node
dissection in renal cell carcinoma.
Eur Urol, 2011,
60, 1212-1220.
Choueiri TK, Tomczak P, et al., KEYNOTE-564 Investigators. Overall Survival with Adjuvant Pembrolizumab in Renal-Cell Carcinoma. N Engl J Med. 2024 Apr 18;390(15):1359-1371. doi: 10.1056/NEJMoa2312695.
DGU; DKG; DKG & Leitlinienprogramm Onkologie Diagnostik, Therapie und Nachsorge des Nierenzellkarzinoms, Langversion 4.02023 https://www.leitlinienprogramm-onkologie.de/leitlinien/nierenzellkarzinom/.
Gill, I. S.; Matin, S. F.; Desai, M. M.; Kaouk, J. H.;
Steinberg, A.; Mascha, E.; Thornton, J.; Sherief, M. H.; Strzempkowski, B.
& Novick, A. C.
Comparative analysis of laparoscopic versus open
partial nephrectomy for renal tumors in 200 patients.
J Urol, 2003,
170, 64-68.
Guinan u.a. 1995 GUINAN, P. D. ; VOGELZANG,
N. J. ; FREMGEN, A. M. ; CHMIEL, J. S. ;
SYLVESTER, J. L. ; SENER, S. F. ; IMPERATO, J. P.:
Renal cell carcinoma: tumor size, stage and survival. Members of the
Cancer Incidence and End Results Committee.
In: J Urol
153 (1995), Nr. 3 Pt 2, S. 901–3
Han u.a. 2003 HAN, K. R. ; BUI, M. H. ;
PANTUCK, A. J. ; FREITAS, D. G. ; LEIBOVICH,
B. C. ; DOREY, F. J. ; ZISMAN, A. ; JANZEN,
N. K. ; MUKOUYAMA, H. ; FIGLIN, R. A. ;
BELLDEGRUN, A. S.:
TNM T3a renal cell carcinoma: adrenal gland involvement is not the
same as renal fat invasion.
In: J Urol
169 (2003), Nr. 3, S. 899–903; discussion 903–4
Hafez u.a. 1999 HAFEZ, K. S. ; FERGANY,
A. F. ; NOVICK, A. C.:
Nephron sparing surgery for localized renal cell carcinoma: impact of
tumor size on patient survival, tumor recurrence and TNM staging.
In: J Urol
162 (1999), Dec, Nr. 6, S. 1930–1933
A. K. Hemal, A. Kumar, R. Kumar, P. Wadhwa, A. Seth, und N. P. Gupta.
Laparoscopic versus open radical nephrectomy for large renal tumors:
a long-term prospective comparison.
J Urol, 177 (3): 862–866, Mar 2007.
Jocham u.a. 2004 JOCHAM, D. ; RICHTER, A. ;
HOFFMANN, L. ; IWIG, K. ; FAHLENKAMP, D. ;
ZAKRZEWSKI, G. ; SCHMITT, E. ; DANNENBERG, T. ;
LEHMACHER, W. ; WIETERSHEIM, J. von ; DOEHN, C.:
Adjuvant autologous renal tumor cell vaccine and risk of tumour
progression in patients with renal-cell carcinoma after radical nephrectomy:
phase III, randomised controlled trial.
In: Lancet
363 (2004), Nr. 9409, S. 594–9
Ljungberg, B.; Albiges, L.; Bensalah, K.; Bex, A.;
Giles, R.; Hora, M.; Kuczyk, M.; Lam, T.; L.Marconi; Merseburger, A.;
Powles, T.; Staehler, M. & Volpe, A.
EAU Guidelines on Renal Cell Carcinoma
2018. https://uroweb.org/guidelines/renal-cell-carcinoma/
Margulis, V.; Master, V. A.; Cost, N. G.; Leibovich, B.
C.; Joniau, S.; Kuczyk, M.; Mulders, P. F.; Kirkali, Z.; Wirth, M. P.;
Hirao, Y.; Rawal, S.; Chong, T. W. & Wood, C. G.
International
consultation on urologic diseases and the European association of urology
international consultation on locally advanced renal cell carcinoma.
Eur
Urol, 2011, 60, 673-683.
Minervini, A.; Serni, S.; Tuccio, A.; Siena, G.;
Vittori, G.; Masieri, L.; Giancane, S.; Lanciotti, M.; Khorrami, S.;
Lapini, A. & Carini, M.
Simple enucleation versus radical
nephrectomy in the treatment of pT1a and pT1b renal cell carcinoma.
Ann
Surg Oncol, 2012, 19, 694-700.
Patard, J.; Pignot, G.; Escudier, B.; Eisen, T.; Bex,
A.; Sternberg, C.; Rini, B.; Roigas, J.; Choueiri, T.; Bukowski, R.;
Motzer, R.; Kirkali, Z.; Mulders, P. & Bellmunt, J.
ICUD-EAU
International Consultation on Kidney Cancer 2010: treatment of metastatic
disease.
Eur Urol, 2011, 60, 684-690.
Poppel, H. V.; Becker, F.; Cadeddu, J. A.; Gill, I. S.;
Janetschek, G.; Jewett, M. A. S.; Laguna, M. P.; Marberger, M.; Montorsi,
F.; Polascik, T. J.; Ukimura, O. & Zhu, G.
Treatment of localised
renal cell carcinoma.
Eur Urol, 2011, 60, 662-672.
Poppel, H. V.; Pozzo, L. D.; Albrecht, W.; Matveev, V.;
Bono, A.; Borkowski, A.; Colombel, M.; Klotz, L.; Skinner, E.; Keane, T.;
Marreaud, S.; Collette, S. & Sylvester, R.
A prospective,
randomised EORTC intergroup phase 3 study comparing the oncologic outcome
of elective nephron-sparing surgery and radical nephrectomy for low-stage
renal cell carcinoma.
Eur Urol, 2011, 59, 543-552.
Robson u.a. 2002 ROBSON, C. J. ; CHURCHILL,
B. M. ; ANDERSON, W.:
The results of radical nephrectomy for renal cell carcinoma. 1969.
In: J Urol
167 (2002), Nr. 2 Pt 2, S. 873–5; discussion 876–7
Sun, M.; Trinh, Q.; Bianchi, M.; Hansen, J.; Hanna, N.;
Abdollah, F.; Shariat, S. F.; Briganti, A.; Montorsi, F.; Perrotte, P. &
Karakiewicz, P. I.
A non-cancer-related survival benefit is associated
with partial nephrectomy.
Eur Urol, 2012, 61,
725-731.
Tsui u.a. 2000 TSUI, K. H. ; SHVARTS, O. ;
SMITH, R. B. ; FIGLIN, R. A. ; DEKERNION, J. B. ;
BELLDEGRUN, A.:
Prognostic indicators for renal cell carcinoma: a multivariate
analysis of 643 patients using the revised 1997 TNM staging criteria.
In: J Urol
163 (2000), Nr. 4, S. 1090–5; quiz 1295
Weight, C. J.; Larson, B. T.; Fergany, A. F.; Gao, T.; Lane, B. R.; Campbell, S. C.; Kaouk, J. H.; Klein, E. A. & Novick, A. C.
Nephrectomy induced chronic renal insufficiency is associated with increased risk of
cardiovascular death and death from any cause in patients with localized
cT1b renal masses.
J Urol, 2010, 183, 1317-1323
Xia L, Wang X, Xu T, Guzzo TJ. Systematic Review and Meta-Analysis of Comparative Studies Reporting Perioperative Outcomes of Robot-Assisted Partial Nephrectomy Versus Open Partial Nephrectomy. J Endourol. 2017 Sep;31(9):893-909. doi: 10.1089/end.2016.0351.
Zini, L.; Perrotte, P.; Capitanio, U.; Jeldres, C.; Shariat, S. F.; Antebi, E.; Saad, F.; Patard, J.; Montorsi, F. &
Karakiewicz, P. I.
Radical versus partial nephrectomy: effect on
overall and noncancer mortality.
Cancer, 2009, 115,
1465-1471
 Deutsche Version: Nierenzellkarzinom
Deutsche Version: Nierenzellkarzinom
Urology-Textbook.com – Choose the Ad-Free, Professional Resource
This website is designed for physicians and medical professionals. It presents diseases of the genital organs through detailed text and images. Some content may not be suitable for children or sensitive readers. Many illustrations are available exclusively to Steady members. Are you a physician and interested in supporting this project? Join Steady to unlock full access to all images and enjoy an ad-free experience. Try it free for 7 days—no obligation.
New release: The first edition of the Urology Textbook as an e-book—ideal for offline reading and quick reference. With over 1300 pages and hundreds of illustrations, it’s the perfect companion for residents and medical students. After your 7-day trial has ended, you will receive a download link for your exclusive e-book.