You are here: Urology Textbook > Kidneys > Renal cell carcinoma > Treatment of advanced renal cell carcinoma
Treatment of Metastatic Renal Cell Carcinoma
- Renal cell carcinoma: Definition and Epidemiology
- Renal cell carcinoma: Pathology
- Renal cell carcinoma: Diagnostic workup
- Renal cell carcinoma: Surgical Treatment
- Renal cell carcinoma: Targeted therapy of Metastases
Prognosis of Metastatic Renal Cell Carcinoma
Motzer from the MSKCC developed accepted criteria for the prognosis of metastatic renal cell carcinoma, which are used for the stratification of patients within trials of immunotherapy (Motzer et al., 2002); see table Motzer criteria. The IMDC score is an update with patient data from modern studies with kinase inhibitors and checkpoint inhibitors (Heng et al., 2013); see table IMDC score for survival time of the risk groups.
.
| Adverse risk factor | Limit |
| Reduced Karnofsky Index | <80% |
| Elevated LDH | >1.5 times the upper limit of normal |
| Low hemoglobin | below the lower limit of normal |
| Elevated corrected calcium | > 10 mg/dl or 2,4 mmol/l |
| Time from nephrectomy to metastases formation | below one year |
.
| Adverse risk factor | Limit |
| Reduced Karnofsky Index | <80% |
| Elevated neutrophils | > upper limit of normal |
| Elevated platelets | > upper limit of normal |
| Low hemoglobin | below the lower limit of normal |
| Elevated corrected calcium | > 10 mg/dl or 2,4 mmol/l |
| Time from nephrectomy to metastases formation | below one year |
Surgical Therapy of Primary Tumor And Metastases
If complete resection of all metastases and the primary tumor is possible, surgical therapy should be done without neoadjuvant therapy. Depending on the location of the metastases (lungs, liver, bones), appropriate surgical specialists are needed. Despite the complete resection of all visible lesions, there is a high risk of recurrence. Still, surgical therapy can delay drug therapy, improve the patient's prognosis, and alleviate local complaints.
The prognosis after removal of a solitary metastasis is less favorable in patients for whom the metastasis already existed at the initial diagnosis. If the metastasis is discovered during the follow-up, the time from initial diagnosis to metastasis is crucial for the prognosis.
Cytoreductive nephrectomy in patients with metastatic renal cell carcinoma:
Cytoreductive nephrectomy is the primary tumor resection before drug therapy for metastases. In addition to palliation of local complaints, nephrectomy before classic immunotherapy leads to an improved survival (Mickisch u.a., 2001a). Laparoscopic cytoreductive nephrectomy offers advantages due to the shorter convalescence time.
Two randomized studies (CARMENA and SURTIME) were carried out for cytoreductive nephrectomy prior to therapy with signal transduction inhibitors. Neither study demonstrated any benefits for cytoreductive nephrectomy before sunitinib (Mejean et al., 2018) (Bex et al., 2019), but only patients with medium or high risk were included.
The current EAU guideline recommends cytoreductive nephrectomy only for patients with a small metastasis volume and a large primary tumor. If the patient responds well to targeted therapy, delayed cytoreductive nephrectomy is an option. There is no indication for cytoreductive nephrectomy in patients with high risk, small primary tumor and high metastatic burden, poor performance status, Bellini duct carcinoma, or sarcomatoid tumors (if the histology is known before surgery).
Checkpoint inhibitors
 |
The activation of immune checkpoint receptors leads to the inhibition and weakening of the cellular immune response. They have a physiological function in preventing autoimmune diseases. Various receptors have been identified: PD-1 receptor (PD for programmed cell death) or CTLA-4 (CTLA for cytotoxic T lymphocyte antigen) on T lymphocytes with corresponding ligands such as PD-L1. Tumors secrete ligands to immune checkpoints to induce immune tolerance. See section also section side effects of checkpoint inhibitors (CPI).
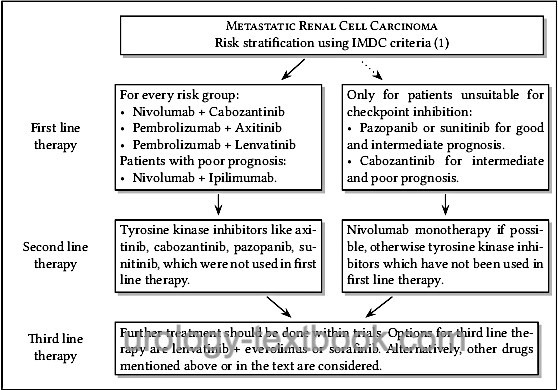
The superiority of the combined therapy (tyrosine kinase inhibitor with immune checkpoint inhibitor) over the monotherapy was demonstrated in several studies. Therapy leads up to 10% to a complete response and often to a significant partial response, partly the treatment effect is delayed. Side effects are substantial (15% grade 3–4, 1.5% treatment-related deaths). A switch to the next option is necessary only if there is clear progress or intolerable side effects (despite adjuvant therapy and dose reduction), see fig. sequential therapy of metastatic renal cell carcinoma.
First-Line Therapy Avelumab combined with Axitinib:
The combination avelumab + axitinib improved progression-free survival (14 vs. 7 months) and response rate (CR 4% vs. 2% and partial response 49% vs. 25%) compared to sunitinib; data for survival have not yet been published (Motzer et al., 2019). The benefit was independent of the IMDC risk classification.
First-Line Therapy Nivolumab combined with Cabozantinib:
The CheckMate-9ER study showed improved progression-free survival (16 vs. 8 months), overall response (56% vs. 27%), complete response (14% vs. 5%) and overall survival (47 vs. 37 months) compared to sunitinib, the benefit was detectable in all risk groups (Choueiri et al., 2021a) (Motzer et al. 2025).
First-Line Therapy Nivolumab combined with Ipilimumab:
The CheckMate-214 study showed significant advantages for nivolumab combined with ipilimumab in progression-free survival and overall survival (56 vs. 38 months after five years follow-up) compared to sunitinib in patients with intermediate and poor prognosis. The combination has also a good response in patients with sarcomatoid renal cell carcinoma (Albiges et al., 2020).
First-Line Therapy Pembrolizumab combined with Axitinib:
Pembrolizumab combined with axitinib leads to an improved progression-free survival, response rate (CR 6% vs. 2%, PR 53% vs. 34%), and overall survival (HR 0.59, 1-year survival rate 90% vs. 79%) (Rini et al., 2019); this was independent of the IMDC risk classification or the PD-L1 expression.
First-Line Therapy Pembrolizumab combined with Lenvatinib:
Pembrolizumab and Lenvatinib improved progression-free survival compared to sunitinib (24 vs. 9 months) and overall survival (HR 0,66) (Motzer et al., 2021).
Second-Line Therapy with Nivolumab:
Nivolumab monotherapy as a second-line therapy after TKI failure (compared to everolimus) improved progression-free survival, response rate (CR + PR: 25% vs. 5%), overall survival (25 vs. 20 months), and showed fewer side effects (Motzer et al., 2015).
Targeted Therapy of Renal Cell Carcinoma With Inhibitors of Signal Transduction
Treatment with inhibitors of signal transduction (smart drugs or targeted therapy) replaced immunotherapy with interferon or interleukin. Inhibitors of signal transduction were standard of treatment before the introduction of checkpoint inhibitors. They are currently administered in combination with checkpoint inhibitors (see above), or as monotherapy when CPI are contraindicated. Inhibitors of signal transduction generate stable disease; complete remission is rare. Favorable prognostic signs are metastases that stabilize or decrease in size and show signs of decreased perfusion in computed tomography.
Targeted molecular agents show a favorable side effect profile in comparison to immunotherapy. Once started, targeted therapy must be continued as long as possible (until progression or intolerable side effects). Sequential therapy of "smart drugs" is followed by a change to the next targeted molecular agents; see flowchart sequential therapy of metastatic renal cell carcinoma.
Axitinib:
Axitinib is a potent inhibitor of VEGF receptor tyrosine kinase. For dosage, pharmacology, and side effects, see chapter pharmacology section Axitinib. Axitinib has been approved for the following indications:
\begin{compactitem} \item First-line therapy pembrolizumab or avelumab combined with axitinib: see above. \item Second-line therapy after progression with sunitinib. A randomized phase 3 trial demonstrated improved progression-free survival (6.7 vs. 4.7 months) with axitinib versus sorafenib \parencite{Rini2011}. \end{compactitem}Bevacizumab:
Bevacizumab is a monoclonal antibody against VEGF and was used in combination with interferon. Bevacizumab is now only of minor therapeutic importance if there are contraindications against first-line therapy.
Cabozantinib:
Cabozantinib is an oral multi-kinase inhibitor of MET, VEGF, and AXL, among others. Cabozantinib has been approved for the following indications:
- First-line therapy nivolumab combined with cabozantinib: see above.
- First-line cabozantinib monotherapy: in medium and high-risk patients with contraindications for CPI. Cabozantinib showed in the CABOSUN trial a higher progression-free survival rate (9 vs. 5 months) in first-line therapy compared to sunitinib (Choueiri et al., 2018).
- Second- and third-line therapy with cabozantinib: Compared to everolimus, progression-free survival (7.4 vs. 3.8 months) and overall survival (21 vs. 19 months) were prolonged in the METEOR trial (Choueiri et al., 2015).
Inhibitors of mTOR:
Everolimus and Temsirolimus are inhibitors of mTOR (mammalian target of rapamycin), a central molecule in the intracellular signal transduction of cell growth, angiogenesis, energy metabolism, and apoptosis (Faivre et al., 2006). Everolimus is an option after failure of first-line therapy with inhibitors of signal transduction (Motzer et al., 2008). Temsirolimus was used to treat high-risk metastatic RCC and is now only of minor importance in third-line therapy.
Lenvatinib:
Lenvatinib is a multi-kinase inhibitor against VEGF receptor kinases, FGFR receptor kinases, PDGF receptor kinases and c-KIT. Lenvatinib has been approved for the following indications:
- First-line therapy pembrolizumab combined with lenvatinib: see above.
- Second-line therapy lenvatinib combined with everolimus: extends progression-free survival (13 vs. 6 months) and overall survival (26 vs. 15 months) compared to everolimus monotherapy in patients after failure of anti-angiogenic therapy (Motzer et al., 2015a).
Pazopanib:
Pazopanib is a tyrosine kinase inhibitor (VEGF receptor, PDGF receptor tyrosine kinase, and c-kit). Pazopanib is an option for first-line therapy in patients with a good prognosis and contraindications for checkpoint inhibitors. Pazopanib is also used for second-line therapy after progression under CPI.
Sorafenib:
Sorafenib is an oral multi-kinase inhibitor which engages in the intracellular signal transduction of cell growth and angiogenesis. Inhibited kinases include RAF kinase, VEGF receptor kinases, PDGF receptor kinase, KIT, and FLT-3 (Escudier et al., 2007a). Sorafenib is a treatment option after first- and second-line therapy has failed.
Sunitinib:
Sunitinib is an oral multi-kinase inhibitor, especially of tyrosine kinases like VEGF and PDGF receptor kinases. Sunitinib is an option for first-line therapy in patients with a good prognosis and contraindications for checkpoint inhibitors. Sunitinib is also used for second-line therapy after progression under CPI.
Tivozanib:
Tivozanib is a tyrosine kinase inhibitor of VEGF receptor kinase with better tolerability compared to sorafenib. Tivozanib is an option for first-line therapy in patients with a good prognosis and contraindications for checkpoint inhibitors. Tivozanib is also used for second-line therapy after progression under CPI. However, the study data is less favorable compared to sunitinib or pazopanib.
Belzutifan:
Belzutifan is a well-tolerated inhibitor of hypoxia-inducible factor-2α (HIF-2α). Belzutifan is approved for treating VHL-associated tumors (Jonasch et al., 2021). Belzutifan is also approved for third-line therapy (after CPI and TKI). Compared with everolism, belzutifan improved progression-free survival 34% vs. 18%, differences in overall survival were insignificant (Choueiri et al., 2024a). There are several Phase 3 trials investigating belzutifan in the first-line treatment of metastatic clear cell renal cell carcinoma.
Immunotherapy With Interferon or Interleukin::
The treatment with interferon-α in combination with interleukin-2 and 5-FU was a treatment option for metastatic renal cell carcinoma. Since the availability of inhibitors of signal transduction and immune checkpoints, treatment with interferon and interleukin is outdated.
Chemotherapy of metastatic renal cell carcinoma:
The following chemotherapy regimens have been tested with only moderate response: gemcitabine and 5-FU, gemcitabine in combination with immunotherapy. No chemotherapeutic regimen is accepted as a standard of care.
Palliative Treatment Options in Metastatic Renal Cell Carcinoma:
Painful bone metastases:
Pain management is standard care. Additional therapeutic options are irradiation, bisphosphonates (zoledronate), denosumab, or surgical stabilization.
Hypercalcemia:
Hypercalcemia is treated with corticosteroids in higher-dose, forced diuresis, saline infusion, and bisphosphonates.
Local pain or bleeding tumor:
Palliative nephrectomy or embolization.
Brain metastases:
Corticosteroids, irradiation (e.g., Gamma knife).
Prognosis of renal cell carcinoma
Natural history of localized renal cell carcinoma:
Renal tumors show, on average, a growth of 3–5 mm per year. Many small tumors do not grow. Metastases from renal tumors smaller than 3–cm are rare.
Clinical stage and prognosis:
See table Robson stage and survival for prognosis and clinical stage. More precise results for the survival probability are possible by considering several risk factors, see stage, size, grade, and necrosis (SSIGN) Score [(Assessing SSIGN Score and Survival and SSIGN Score)].
Venous invasion:
Almost 40% of patients with venous invasion are not suffering from metastases (pN0 and M0) and may be cured by surgery. Patients with pN0 M0 have a 5-year survival rate of 70%. 26% have lymph node metastases, 54% have distant metastases.
Grading and survival:
Five-year survival rates depending on grading: G1 (89%), G2 (65%), G3 (46%).
Prognosis of renal cell carcinoma with lymph node metastasis:
Five-year survival rate 5–30%.
Prognosis of renal cell carcinoma with systemic metastasis:
Metastatic renal cell cancer is a very aggressive disease. Patients with metastases at initial diagnosis have a poor prognosis and usually die within the first year. The interval from the nephrectomy to the onset of metastases is important for prognosis, as it provides information on the rate of progression of the systemic disease. Patients with pulmonary metastases have the best prognosis. In addition to metastases in other locations, the following factors indicate a poor prognosis: low Karnofsky index, cancer-related anemia, abnormal high corrected serum calcium, elevated LDH, elevated AP, elevated platelets, and elevated neutrophils. See tab. IMDC Score for mean survival time under treatment with TKI and CPI.
Prognosis of renal cell carcinoma with brain metastases:
Median survival of 7 months; prognostic factors are Karnofsky index and number of metastases.
| RCC surgical therapy | Index | Wilms-Tumor |
Index: 1–9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
References
L. Albiges, N. M. Tannir, M. Burotto, D. McDermott, E. R. Plimack, and R. J. Motzer, “Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial.,” ESMO open, vol. 5, no. 6, p. e001079, 2020.
Choueiri, T. K.; Escudier, B.; Powles, T.; Mainwaring,
P. N.; Rini, B. I.; Donskov, F.; Hammers, H.; Hutson, T. E.; Lee, J.-L.;
Peltola, K.; Roth, B. J.; Bjarnason, G. A.; Géczi, L.; Keam, B.; Maroto,
P.; Heng, D. Y. C.; Schmidinger, M.; Kantoff, P. W.; Borgman-Hagey, A.;
Hessel, C.; Scheffold, C.; Schwab, G. M.; Tannir, N. M.; Motzer, R. J. & ,
M. E. T. E. O. R. I.
Cabozantinib versus Everolimus in Advanced
Renal-Cell Carcinoma.
N Engl J Med, 2015, 373,
1814-1823.
T. K. Choueiri et al., “Belzutifan versus Everolimus for Advanced Renal-Cell Carcinoma.,” NEJM, vol. 391, no. 8, pp. 710–721, 2024.
DGU; DKG; DKG & Leitlinienprogramm Onkologie Diagnostik, Therapie und Nachsorge des Nierenzellkarzinoms, Langversion 4.02023 https://www.leitlinienprogramm-onkologie.de/leitlinien/nierenzellkarzinom/.
Escudier u.a. 2007 ESCUDIER, Bernard ; EISEN,
Tim ; STADLER, Walter M. ; SZCZYLIK, Cezary ;
OUDARD, Stéphane ; SIEBELS, Michael ; NEGRIER,
Sylvie ; CHEVREAU, Christine ; SOLSKA, Ewa ;
DESAI, Apurva A. ; ROLLAND, Frédéric ; DEMKOW,
Tomasz ; HUTSON, Thomas E. ; GORE, Martin ;
FREEMAN, Scott ; SCHWARTZ, Brian ; SHAN, Minghua ;
SIMANTOV, Ronit ; BUKOWSKI, Ronald M. ; GROUP, T.
A. R. G. E. T. S.:
Sorafenib in advanced clear-cell renal-cell carcinoma.
In: N Engl J Med
356 (2007), Jan, Nr. 2, S. 125–134
B. Escudier, T. K. Choueiri, S. Oudard, C. Szczylik, S. Negrier, A. Ravaud,
C. Chevreau, P. Venner, P. Champagne, D. Croteau, E. Dupont, C. Hariton, and
R. M. Bukowski.
Prognostic factors of metastatic renal cell carcinoma after failure
of immunotherapy: new paradigm from a large phase iii trial with shark
cartilage extract ae 941.
J Urol, 178 (5): 1901–1905, Nov 2007.
doi: rm10.1016/j.juro.2007.07.035.
URL https://dx.doi.org/10.1016/j.juro.2007.07.035.
B. Escudier, A. Pluzanska, P. Koralewski, A. Ravaud, S. Bracarda, C. Szczylik,
C. Chevreau, M. Filipek, B. Melichar, E. Bajetta, V. Gorbunova, J. O. Bay,
I. Bodrogi, A. Jagiello-Gruszfeld, und N. Moore.
Bevacizumab plus interferon alfa-2a for treatment of metastatic renal
cell carcinoma: a randomised, double-blind phase iii trial.
Lancet, 370 (9605): 2103–2111, Dec 2007.
S. Faivre, G. Kroemer, and E. Raymond.
Current development of mtor inhibitors as anticancer agents.
Nat Rev Drug Discov, 5 (8): 671–688, Aug
2006.
doi: rm10.1038/nrd2062.
URL https://dx.doi.org/10.1038/nrd2062.
Frank u.a. 2003 FRANK, I. ; BLUTE, M. L. ;
CHEVILLE, J. C. ; LOHSE, C. M. ; WEAVER, A. L. ;
LEIBOVICH, B. C. ; ZINCKE, H.:
A multifactorial postoperative surveillance model for patients with
surgically treated clear cell renal cell carcinoma.
In: J Urol
170 (2003), Nr. 6 Pt 1, S. 2225–32
Gold u.a. 1996 GOLD, P. J. ; FEFER, A. ;
THOMPSON, J. A.:
Paraneoplastic manifestations of renal cell carcinoma.
In: Semin Urol Oncol
14 (1996), Nr. 4, S. 216–22
Heng, D. Y. C.; Wells, J. C.; Rini, B. I.; Beuselinck,
B.; Lee, J.-L.; Knox, J. J.; Bjarnason, G. A.; Pal, S. K.;
Kollmannsberger, C. K.; Yuasa, T.; Srinivas, S.; Donskov, F.; Bamias, A.;
Wood, L. A.; Ernst, D. S.; Agarwal, N.; Vaishampayan, U. N.; Rha, S. Y.;
Kim, J. J. & Choueiri, T. K.
Cytoreductive nephrectomy in patients
with synchronous metastases from renal cell carcinoma: results from the
international metastatic renal cell carcinoma database consortium.
Eur Urol 2014,
66, 704-710.
G. Hudes, M. Carducci, P. Tomczak, J. Dutcher, R. Figlin, A. Kapoor,
E. Staroslawska, J. Sosman, D. McDermott, I. Bodrogi, Z. Kovacevic,
V. Lesovoy, I. G. H. Schmidt-Wolf, O. Barbarash, E. Gokmen, T. O’Toole,
S. Lustgarten, L. Moore, R. J. Motzer, and G. A. Trial.
Temsirolimus, interferon alfa, or both for advanced renal-cell
carcinoma.
N Engl J Med, 356 (22): 2271–2281, May 2007.
E. Jonasch et al., “Belzutifan for Renal Cell Carcinoma in von Hippel-Lindau Disease.,” NEJM, vol. 385, no. 22, pp. 2036–2046, 2021, doi: 10.1056/NEJMoa2103425.
Kattan u.a. 2001 KATTAN, M. W. ; REUTER, V. ; MOTZER, R. J. ; KATZ, J. ; RUSSO, P.: A postoperative prognostic nomogram for renal cell carcinoma.In: J Urol
166 (2001), Nr. 1, S. 63–7
Rana R. McKay u.a. Impact of angiotensin system inhibitors (ASI) on outcomes in patients (pts) with metastatic renal cell carcinoma (mRCC): Results from a pooled clinical trials database. J Clin Oncol 32, 2014 (suppl 4; abstr 437).
B. C. Leibovich, M. L. Blute, J. C. Cheville, C. M. Lohse, I. Frank, E. D.
Kwon, A. L. Weaver, A. S. Parker, und H. Zincke.
Prediction of progression after radical nephrectomy for patients with
clear cell renal cell carcinoma: a stratification tool for prospective
clinical trials.
Cancer, 97 (7): 1663–1671, Apr 2003.
doi: rm10.1002/cncr.11234.
URL https://dx.doi.org/10.1002/cncr.11234.
Ljungberg, B.; Albiges, L.; Bensalah, K.; Bex, A.;
Giles, R.; Hora, M.; Kuczyk, M.; Lam, T.; L.Marconi; Merseburger, A.;
Powles, T.; Staehler, M. & Volpe, A.
EAU Guidelines on Renal Cell Carcinoma
2018. https://uroweb.org/guidelines/renal-cell-carcinoma/
Mancuso und Sternberg 2005 MANCUSO, A. ;
STERNBERG, C. N.:
What’s new in the treatment of metastatic kidney cancer?
In: BJU Int
95 (2005), Nr. 9, S. 1171–80
Mickisch, G. H.; Garin, A.; van Poppel, H.; de Prijck,
L.; Sylvester, R.; , E. O. f. R. & of Cancer (EORTC) Genitourinary Group,
T.
Radical nephrectomy plus interferon-alfa-based immunotherapy
compared with interferon alfa alone in metastatic renal-cell carcinoma: a
randomised trial.
Lancet, 2001, 358, 966-970.
Motzer und Bukowski 2006 MOTZER, Robert J. ;
BUKOWSKI, Ronald M.:
Targeted therapy for metastatic renal cell carcinoma.
In: J Clin Oncol
24 (2006), Dec, Nr. 35, S. 5601–5608. -
URL https://dx.doi.org/10.1200/JCO.2006.08.5415
Motzer u.a. 2007 MOTZER, Robert J. ; HUTSON,
Thomas E. ; TOMCZAK, Piotr ; MICHAELSON, M. D. ;
BUKOWSKI, Ronald M. ; RIXE, Olivier ; OUDARD,
Stéphane ; NEGRIER, Sylvie ; SZCZYLIK, Cezary ;
KIM, Sindy T. ; CHEN, Isan ; BYCOTT, Paul W. ;
BAUM, Charles M. ; FIGLIN, Robert A.:
Sunitinib versus interferon alfa in metastatic renal-cell carcinoma.
In: N Engl J Med
356 (2007), Jan, Nr. 2, S. 115–124. -
URL https://dx.doi.org/10.1056/NEJMoa065044
Motzer, R. J.; Escudier, B.; McDermott, D. F.; George,
S.; Hammers, H. J.; Srinivas, S.; Tykodi, S. S.; Sosman, J. A.; Procopio,
G.; Plimack, E. R.; Castellano, D.; Choueiri, T. K.; Gurney, H.; Donskov,
F.; Bono, P.; Wagstaff, J.; Gauler, T. C.; Ueda, T.; Tomita, Y.; Schutz,
F. A.; Kollmannsberger, C.; Larkin, J.; Ravaud, A.; Simon, J. S.; Xu,
L.-A.; Waxman, I. M.; Sharma, P. & CheckMate025
Nivolumab versus
Everolimus in Advanced Renal-Cell Carcinoma.
New Engl J Med 2015, 373,
1803-1813.
Motzer, R. J.; Hutson, T. E.; Glen, H.;
Michaelson, M. D.; Molina, A.; Eisen, T.; Jassem, J.; Zolnierek, J.;
Maroto, J. P.; Mellado, B.; Melichar, B.; Tomasek, J.; Kremer, A.; Kim,
H.-J.; Wood, K.; Dutcus, C. & Larkin, J.
Lenvatinib, everolimus,
and the combination in patients with metastatic renal cell carcinoma: a
randomised, phase 2, open-label, multicentre trial.
The Lancet.
Oncology, 2015a, 16, 1473-1482.
R. J. Motzer, B. Escudier, S. Oudard, T. E. Hutson, C. Porta, S. Bracarda,
V. Gruenwald, J. A. Thompson, R. A. Figlin, N. Hollaender, G. Urbanowitz,
W. J. Berg, A. Kay, D. Lebwohl, und A. Ravaud.
Efficacy of everolimus in advanced renal cell carcinoma: a
double-blind, randomised, placebo-controlled phase iii trial.
Lancet, Jul 2008.
R. J. Motzer et al.“Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial.,” Journal of clinical oncology : official journal of the American Society of Clinical Oncology, vol. 31, no. 30, pp. 3791–3799, 2013.
Motzer RJ, Escudier B, Choueiri TK et al. Final analysis of nivolumab plus cabozantinib for advanced renal cell carcinoma from the randomized phase III CheckMate 9ER trial. Ann Oncol. 2025 Sep 23:S0923-7534(25)04714-3. doi: 10.1016/j.annonc.2025.09.006.
S. Negrier, D. Perol, A. Ravaud, C. Chevreau, J. O. Bay, R. Delva, E. Sevin,
A. Caty, und B. Escudier.
Medroxyprogesterone, interferon alfa-2a, interleukin 2, or
combination of both cytokines in patients with metastatic renal carcinoma of
intermediate prognosis: results of a randomized controlled trial.
Cancer, 110 (11): 2468–2477, Dec 2007.
Powles, T.; Albiges, L.; Staehler, M.; Bensalah,
K.; Dabestani, S.; Giles, R. H.; Hofmann, F.; Hora, M.; Kuczyk, M. A.;
Lam, T. B.; Marconi, L.; Merseburger, A. S.; Fernández-Pello, S.; Tahbaz,
R.; Volpe, A.; Ljungberg, B. & Bex, A.
Updated European Association
of Urology Guidelines Recommendations for the Treatment of First-line
Metastatic Clear Cell Renal Cancer.
European urology, 2017
Rini, B. I.; Escudier, B.; Tomczak, P.; Kaprin, A.; Szczylik, C.; Hutson, T. E.; Michaelson, M. D.; Gorbunova, V. A.; Gore, M. E.; Rusakov, I. G.; Negrier, S.; Ou, Y.; Castellano, D.; Lim, H. Y.; Uemura, H.; Tarazi, J.; Cella, D.; Chen, C.; Rosbrook, B.; Kim, S. & Motzer, R. J. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial.
Lancet, 2011, 378, 1931-1939.
Sternberg, C. N.; Davis, I. D.; Mardiak, J.; Szczylik, C.; Lee, E.; Wagstaff, J.; Barrios, C. H.; Salman, P.; Gladkov, O. A.; Kavina, A.; Zarbá, J. J.; Chen, M.; McCann, L.; Pandite, L.; Roychowdhury, D. F. & Hawkins, R. E. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial.
2010, 28, 1061-1068.
Weight, C. J.; Larson, B. T.; Fergany, A. F.; Gao, T.; Lane, B. R.; Campbell, S. C.; Kaouk, J. H.; Klein, E. A. & Novick, A. C. Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses.
J Urol, 2010, 183, 1317-1323
Zini, L.; Perrotte, P.; Capitanio, U.; Jeldres, C.; Shariat, S. F.; Antebi, E.; Saad, F.; Patard, J.; Montorsi, F. & Karakiewicz, P. I. Radical versus partial nephrectomy: effect on overall and noncancer mortality.
Cancer, 2009, 115, 1465-1471
Zisman u.a. 2002 ZISMAN, A. ; PANTUCK, A. J. ;
WIEDER, J. ; CHAO, D. H. ; DOREY, F. ;
SAID, J. W. ; DEKERNION, J. B. ; FIGLIN, R. A. ;
BELLDEGRUN, A. S.:
Risk group assessment and clinical outcome algorithm to predict the
natural history of patients with surgically resected renal cell carcinoma.
In: J Clin Oncol
20 (2002), Nr. 23, S. 4559–66
 Deutsche Version: Nierenzellkarzinom
Deutsche Version: Nierenzellkarzinom
Urology-Textbook.com – Choose the Ad-Free, Professional Resource
This website is designed for physicians and medical professionals. It presents diseases of the genital organs through detailed text and images. Some content may not be suitable for children or sensitive readers. Many illustrations are available exclusively to Steady members. Are you a physician and interested in supporting this project? Join Steady to unlock full access to all images and enjoy an ad-free experience. Try it free for 7 days—no obligation.
New release: The first edition of the Urology Textbook as an e-book—ideal for offline reading and quick reference. With over 1300 pages and hundreds of illustrations, it’s the perfect companion for residents and medical students. After your 7-day trial has ended, you will receive a download link for your exclusive e-book.