You are here: Urology Textbook > Kidneys > Renal cell carcinoma > Pathology
Pathology and TNM Stages of Renal Cell Carcinoma
- Renal cell carcinoma: Definition and Epidemiology
- Renal cell carcinoma: Pathology
- Renal cell carcinoma: Diagnostic workup
- Renal cell carcinoma: Surgical Treatment
- Renal cell carcinoma: Targeted therapy of advanced disease
TNM Tumor Stages [UICC 2017]
T1:
Tumor ≤7 cm in greatest dimension, confined to the kidney.
- T1a: Tumor size ≤4 cm
- T1b: Tumor size 4–7 cm
T2:
Tumor >7 cm in greatest dimension, confined to the kidney.
- T2a: Tumor size 7–10 cm.
- T2b: Tumor size >10 cm
T3:
Tumor extends into major veins or perinephric tissues but not into the ipsilateral adrenal gland and not beyond the renal fascia.
- T3a: Tumor extension into major renal veins or infiltration of perirenal adipose tissue
- T3b: Tumor extension into the vena cava below the diaphragm
- T3c: Tumor extension into the vena cava above the diaphragm or invasion of the wall of the vena cava
T4:
Tumor invades beyond the renal fascia or shows contiguous extension into the ipsilateral adrenal gland.
N:
Lymph node involvement
- N0: No regional lymph node metastases
- N1: Metastasis in regional lymph nodes
M:
Distant metastasis
- M0: No distant metastasis
- M1: Distant metastasis are present
G:
Grading
- G1: well differentiated
- G2: moderately differentiated
- G3: poorly differentiated
- G4: undifferentiated
Stage Grouping [UICC]
- Stage I: T1 N0 M0
- Stage II: T2 N0 M0
- Stage III: T3 N0 M0 or T1–T3 N1 M0
- Stage IV: T4 N0–1 M0 or T1–4 N0–1 M1
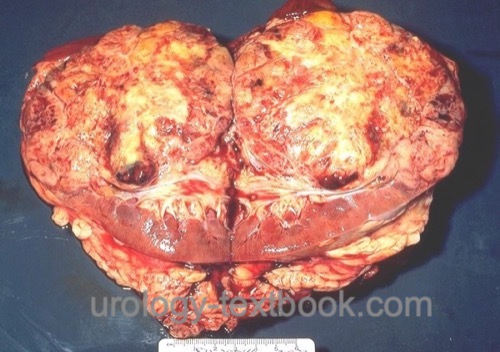 |
Macroscopic Pathology of Renal Cell Carcinoma
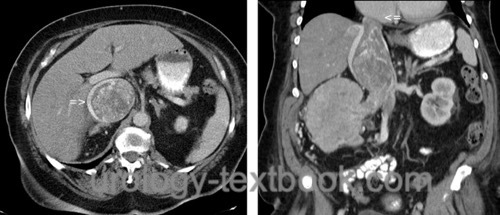
The classic clear cell renal cell carcinoma has a yellow-brown cut surface and is inhomogeneous due to hemorrhage and necrosis [fig. surgical specimen kidney with renal cell carcinoma]. Macroscopically, it is relatively well separated from the normal renal tissue, but there is a risk of forming microscopic tumor satellites. Only sarcomatoid tumors always present with infiltrative growth. Other macroscopic manifestations include cystic growth patterns or calcifications (10–20%). Invasion of the renal veins occurs in 10%, and by continuous tumor growth, a tumor thrombus may extend via the vena cava inferior until the right atrium [fig. large tumor thrombus in the vena cava inferior].
 |
Microscopic Pathology of Renal Cell Carcinoma
The current classification of renal cell carcinoma considers morphological, genetic, and prognostic differences (Moch et al., 2016). Since subtypes differ in molecular carcinogenesis, variable therapeutic responses to signal transduction inhibitors are likely (Algaba et al., 2011).
Clear cell renal cell carcinoma (70–80%):
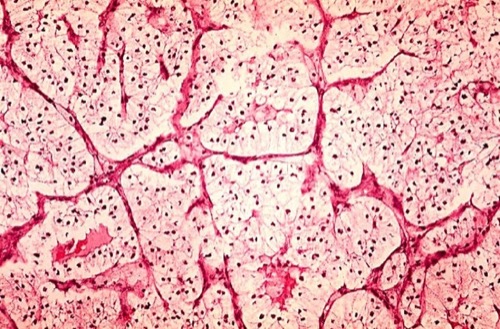
Clear cell renal cell carcinoma macroscopically has a yellow-brown cut surface. The cells are derived from the proximal convoluted tubule. The rich content of glycogen and fat in the cytoplasm of the cells produces a clear appearance in conventional staining [fig. histology of renal cell carcinoma]. But there are also eosinophilic, sarcomatoid (1-5 %), and mixed differentiation patterns. Mutations of the VHL gene are detectable in 75% of clear cell RCC.
 |
Papillary renal cell carcinoma (10%):
Papillary renal cell carcinoma presents with basophilic (type 1) or eosinophilic (type 2) cells that form papillary or tubular patterns. The cells are derived from the proximal convoluted tubule. In contrast to clear cell renal cell carcinoma, papillary RCC is not well vascularized. The prognosis is better if localized, but worse if metastasized in comparison to clear cell renal cell carcinoma (Steffens et al., 2012).
Chromophobe renal cell carcinoma (5%):
Chromophobe renal cell carcinoma is derived from the cortical portion of the collecting duct. The lack of staining of the cytoplasm in the tumor cells was eponymous. Numerous microvesicles containing a mucopolysaccharide are visible with the electron microscope. Chromophobe renal cell carcinoma is the malignant variant of Onkozytoma. The prognosis is better than clear cell RCC.
Collecting duct carcinoma or Bellini duct cancer (1%):
Collecting duct carcinoma shows a mixed image of dilated tubules and papillary structures. The prognosis is poor.
Renal medullary carcinoma (less than 1%):
Renal medullary carcinoma occurs almost only in sickle cell disease and arises from the epithelium of the calyces. Histology and prognosis are similar to collecting duct carcinoma; some authors consider it as a subtype of the collecting duct carcinoma.
Mucinous tubular and spindle cell carcinoma (less than 1%):
Mucinous tubular and spindle cell carcinoma is considered as a low-grade entity and occurs mainly in women under 60. Some authors consider it to be a subtype of papillary renal cell carcinoma.
Multilocular cystic renal neoplasia with low malignant potential:
Cystic tumor with low-grade tumor cells, in numerous studies without proven metastasis, therefore the old term multilocular cystic renal cell carcinoma is no longer used.
MiT Family translocation renal cell carcinoma:
Various translocations with activation of the MiT family cause MiT family translocation renal cell carcinoma. Known translocation affect chromosome Xp11.2 or t(6;11). Mainly children and adolescents are affected; this tumor type is usually detected at an advanced stage.
Succinate Dehydrogenase–Deficient Renal Cell Carcinoma:
SDH-deficient RCC is characterized by a loss of SDHB expression, a marker of mitochondrial dysfunction. The tumors are solid with a brown cut surface and cytoplasmic vacuoles. It affects mainly young adults, and most patients have a germline mutation. The prognosis is favorable.
Not classifiable renal cell carcinomas:
4–7% renal cell carcinomas are large, undifferentiated, and cannot be classified. The prognosis is poor (median survival 4–5 months).
Oncocytoma (5%):
Please see section oncocytoma.
Metastasis of Renal Cell Carcinoma
RCC most commonly metastasizes into the lung, soft tissues (lymph nodes, muscles), bone, liver, CNS, and skin (in descending order of frequency).
| Kidney cancer | Index | RCC Symptoms |
Index: 1–9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
References
Algaba, F.; Akaza, H.; López-Beltrán, A.; Martignoni,
G.; Moch, H.; Montironi, R. & Reuter, V.
Current pathology keys of
renal cell carcinoma.
Eur Urol, 2011, 60, 634-643.
Badeloe, S. & Frank, J.
Clinical and molecular
genetic aspects of hereditary multiple cutaneous leiomyomatosis.
Eur
J Dermatol, 2009, 19, 545-551
DGU; DKG; DKG & Leitlinienprogramm Onkologie Diagnostik, Therapie und Nachsorge des Nierenzellkarzinoms, Langversion 4.02023 https://www.leitlinienprogramm-onkologie.de/leitlinien/nierenzellkarzinom/.
Eble, J. N.; Sauter, G.; Epstein, J. I. & Sesterhenn,
I. A. (ed.)
World Health Organization classification of tumours.
Pathology and genetics of tumours of the urinary system and male genital
organs
IARC Press, 2004.
Hock u.a. 2002 HOCK, L. M. ; LYNCH, J. ;
BALAJI, K. C.:
Increasing incidence of all stages of kidney cancer in the last 2
decades in the United States: an analysis of surveillance, epidemiology and
end results program data.
In: J Urol
167 (2002), Nr. 1, S. 57–60
Ljungberg, B.; Albiges, L.; Bensalah, K.; Bex, A.;
Giles, R.; Hora, M.; Kuczyk, M.; Lam, T.; L.Marconi; Merseburger, A.;
Powles, T.; Staehler, M. & Volpe, A.
EAU Guidelines on Renal Cell Carcinoma
2018. https://uroweb.org/guidelines/renal-cell-carcinoma/
Ljungberg, B.; Campbell, S. C.; Cho, H. Y.; Jacqmin,
D.; Lee, J. E.; Weikert, S. & Kiemeney, L. A.
The epidemiology of
renal cell carcinoma.
Eur Urol, 2011, 60, 615-621.
Menko, F. H.; van Steensel, M. A. M.; Giraud, S.;
Friis-Hansen, L.; Richard, S.; Ungari, S.; Nordenskjöld, M.; Hansen, T.
V.; Solly, J.; Maher, E. R. & Consortium, E. B.
Birt-Hogg-Dubé
syndrome: diagnosis and management.
Lancet Oncol, 2009,
10, 1199-1206.
Steffens, S.; Janssen, M.; Roos, F. C.; Becker, F.;
Schumacher, S.; Seidel, C.; Wegener, G.; Thüroff, J. W.; Hofmann, R.;
Stöckle, M.; Siemer, S.; Schrader, M.; Hartmann, A.; Kuczyk, M. A.;
Junker, K. & Schrader, A. J.
Incidence and long-term prognosis of
papillary compared to clear cell renal cell carcinoma - a multicentre study.
Eur
J Cancer, 2012, 48, 2347-2352.
Storkel u.a. 1997 STORKEL, S. ; EBLE, J. N. ;
ADLAKHA, K. ; AMIN, M. ; BLUTE, M. L. ;
BOSTWICK, D. G. ; DARSON, M. ; DELAHUNT, B. ;
ICZKOWSKI, K.:
Classification of renal cell carcinoma: Workgroup No. 1. Union
Internationale Contre le Cancer (UICC) and the American Joint Committee on
Cancer (AJCC).
In: Cancer
80 (1997), Nr. 5, S. 987–9
Verine, J.; Pluvinage, A.; Bousquet, G.; Lehmann-Che,
J.; de Bazelaire, C.; Soufir, N. & Mongiat-Artus, P.
Hereditary
renal cancer syndromes: an update of a systematic review.
Eur Urol, 2010,
58, 701-710.
 Deutsche Version: Nierenzellkarzinom
Deutsche Version: Nierenzellkarzinom
Urology-Textbook.com – Choose the Ad-Free, Professional Resource
This website is designed for physicians and medical professionals. It presents diseases of the genital organs through detailed text and images. Some content may not be suitable for children or sensitive readers. Many illustrations are available exclusively to Steady members. Are you a physician and interested in supporting this project? Join Steady to unlock full access to all images and enjoy an ad-free experience. Try it free for 7 days—no obligation.
New release: The first edition of the Urology Textbook as an e-book—ideal for offline reading and quick reference. With over 1300 pages and hundreds of illustrations, it’s the perfect companion for residents and medical students. After your 7-day trial has ended, you will receive a download link for your exclusive e-book.