You are here: Urology Textbook > Surgery (procedures) > Laparoscopy
Laparoscopy and Robotic Surgery in Urology
History of Laparoscopy
Diagnostic laparoscopy:
After Nitsche invented the cystoscope in 1879, Kelling used this cystoscope in 1901 for the first diagnostic laparoscopy in animals. Jacobaeus performed the first diagnostic laparoscopy in humans in 1910. Diagnostic laparoscopy remained until the mid-20th century in the hands of specialists in internal medicine, who further improved the technique. Important steps of progress were the invention of the Veress needle, CO2-insufflation, trocars, powerful light sources, and improved rod lens systems.
 |
Operative Laparoscopy:
The gynecologist Semm is acknowledged as the founder of laparoscopic surgery. He developed insufflators, loop ligation, and various instruments, and many laparoscopic procedures were introduced by Semm, e.g., first uterine fibroids (1972 ), ovariectomy (1977 ), and laparoscopic appendectomy (1980). After the establishment of laparoscopy in the surgical community (first laparoscopic cholecystectomy by Mouret 1987), urologists slowly began to use laparoscopy. Table laparoscopic history provides information about the timeline of laparoscopic urologic surgery.
| Year | Author | Procedure |
| 1976 | Cortesi | Laparoscopy for cryptorchidism |
| 1990 | Sanchez-de-Badajoz | Varicocelectomy |
| 1991 | Schuessler | pelvic lymphadenectomy |
| 1991 | Clayman | Nephrectomy |
| 1992 | Schuessler | Prostatectomy |
| 1992 | Parra | Cystectomy |
| 2000 | Binder | Robotic-assisted prostatectomy |
| 2003 | Menon | Robotic-assisted cystectomy |
Robotic Laparoscopy:
Another milestone in the development of laparoscopic surgical techniques is the development of robotic-assisted laparoscopy. Intuitive Surgical is the first provider (da Vinci Surgical console) and has the largest market share. New providers with small market shares are Medtronic (Hugo), Cambridge Medical Robotics (Versius), Asensus Surgical (Senhance), and Johnson & Johnson (Ottava). The console consists of two components: a control console for the surgeon and a patient-side robotic unit. The da Vinci Surgical console has 3 to 4 arms that carry the surgical instruments and the camera unit. The camera unit provides an accurate stereoscopic view of the surgical field with magnification. The surgical unit enhances dexterity, precision, and ergonomic comfort. The articulated instruments allow movements that are not possible with rigid standard instruments of laparoscopy. In sum, robotic surgery enables more complex minimally invasive procedures involving complex dissection or reconstruction (partial nephrectomy, prostatectomy, and cystectomy with urinary diversion).
Advantages:
Due to the mechanic improvements, complex reconstructive procedures become feasible with a laparoscopic approach. Laparoscopic prostatectomy, previously practiced only by skilled laparoscopic surgeons, became a new surgical standard in several countries with the help of the robotic-assisted technique. The robotic-assisted technique also allows complex partial kidney resections and radical cystectomy with various forms of urinary diversion with the advantages of a laparoscopic approach.
Disadvantages:
The main disadvantages are the enormous costs, which include the investment, the maintenance contract with the provider, and the need for expensive disposable instruments. Further disadvantages are additional operating time for docking the console to the patient, no tactile feedback, and, very rarely, mechanical malfunctions with potential damage to the patient.
Indications for Laparoscopy in Urology
Diagnostic laparoscopy:
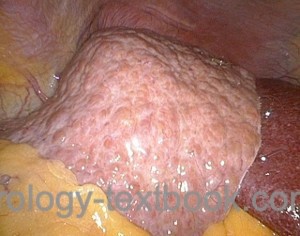
Diagnostic laparoscopy is rarely indicated in urology, e.g., diagnosing the testicular location in cryptorchidism.
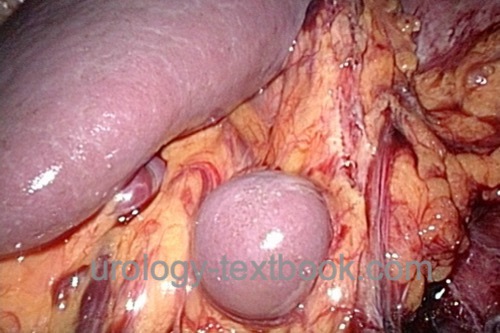 |
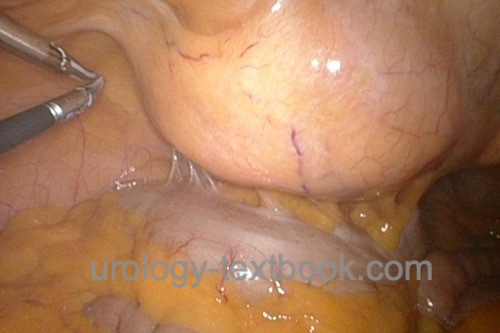 |
 |
Operative laparoscopy:
The spectrum of laparoscopic surgery in urology is continuously growing. However, the individual surgeon's level of training is vital for success. Frequently performed laparoscopic operations are pelvic or retroperitoneal lymph node dissection, nephrectomy, partial nephrectomy, adrenalectomy, prostatectomy, and surgery for UPJ obstruction. With the help of robotic-assisted laparoscopy, complicated reconstructive procedures in urology are becoming possible, such as complex partial nephrectomy of hilar tumors or cystectomy with intracorporal urinary diversion.
Contraindications of Laparoscopy
Underlying Diseases:
- Severe COPD
- Severe heart failure
- Peritonitis
- Ileus
- Active bleeding
- Large aortic aneurysm
Previous Surgery:
After uncomplicated appendectomy, cholecystectomy, or hysterectomy, laparoscopy is often possible and successful. If severe adhesions are present, laparoscopy should not be forced, and a secure open approach is advisable. Another alternative is the retroperitoneoscopic approach.
Surgical Technique of Laparoscopy and Robotic Surgery
Preoperative Preparations:
- Recommend a low fiber diet two days before surgery and a clear liquid diet on the preoperative day to improve the intraabdominal space.
- Administer an enema the night before surgery.
- Perioperative placement of a urinary catheter and gastric tube.
- Perioperative antibiotic prophylaxis is needed if risk factors for surgical site infections are present or if the urinary tract is planned to be opened.
- Insert a transurethral catheter and gastric feeding tube during surgery.
Patient Positioning for Renal Laparoscopic Procedures:
The patient is positioned in a lateral decubitus position at an angle of 45 degrees and with mild lumbar hyperextension. A vacuum mattress enables a secure fixation of the patient, even if the operation table has to be tilted.
Patient Positioning for Pelvic Laparoscopic Procedures:
The patient is in a supine position with mild lumbar hyperextension, the arms next to the body. A vacuum mattress enables a secure patient fixation, even if a steep Trendelenburg positioning is needed.
Establishment of the Pneumoperitoneum:
Carbon dioxide (CO2) is used to distense the abdominal cavity with a working pressure usually 10–14 mmHg for adults and 6–10 mmHg for children. The establishment of the pneumoperitoneum can be done via a closed approach (Veress needle) or via an open technique for the first trocar, which is more secure and recommended (Hasson et al., 2000).
Open technique for first-trocar placement:
A 2 cm long incision is done next to the umbilicus. Incise the ventral fascia of the rectus sheath, and use two small Langenbeck retractors for exposure. Place stay sutures at the corners of the incision if a Hasson trocar is used. Bluntly split of the rectus abdominis muscle. Incise the transversalis fascia and the peritoneum. Palpate for adherent bowel loops before placing the first trocar (Hasson trocar or trocar with balloon fixation). Connect the insufflator to the trocar using a working pressure of 12 mmHg initially with maximum gas flow. The filling of the abdominal cavity through the trocar is fast and compensates a little for the longer dissection time of the open technique.
Closed establishment of the pneumoperitoneum:
A 1 cm long incision is done next to the umbilicus. Lift the abdominal wall and advance the Veress needle through the abdominal wall layers into the peritoneal cavity. The Veress needle is a spring-loaded device; if the needle enters the peritoneal cavity, the sharp needle is hidden by the dull inner stylet protecting the bowel, audible by a clicking sound. Check the correct position of the Veress needle using a 20 ml syringe filled with 10 ml of saline:
- Aspiration: neither fluid (blood, bile, or stool) nor air can be aspirated.
- Injection: 10 ml of saline can be injected without resistance, which may not be aspirated afterward.
- The needle may be advanced and rotated without significant resistance.
The insufflator is connected to fill the abdominal cavity with CO2 with a working pressure of 12 mmHg. With a good position of the Veress needle, the gas flow should be above 1 l/min. An increase in the working pressure or a low flow rate suggests an incorrect position of the Veress needle or the complete filling of the abdominal cavity. The volume to fill the abdominal cavity is around 3–5 l, depending on working pressure, body weight, and muscle relaxation. The optic trocar is gently advanced into the gas-filled peritoneal cavity. Modern trocars allow the advancement of the first trocar with optical control through the laparoscope.
Laparoscopy:
Perform a systematic inspection of the entire abdominal cavity.
Working trocars:
Place the working trocars under direct vision; the positions depend on the planned operation. An ideal trocar position leads to instruments meeting in the operative field at a right angle.
Laparoscopic Instruments:
In principle, laparoscopic instruments consist of a handle (locked or not locked), an isolated instrument shaft (normal length 33–36 cm) and the jaw insert connected through the instrument shaft to the handle.
Scissors:
Most often a pair of scissors with curved branches are used (Metzenbaum scissors). It is possible to connect monopolar current for simultaneous coagulation. Scissors with bipolar coagulation are also available.
Ultrasonic scalpel:
The ultrasound scalpel is an alternative cutting instrument. It uses high-frequency vibration of the dull scalpel, which cuts and coagulates simultaneously. The ultrasonic scalpel (harmonic scalpel) causes less lateral thermal damage than monopolar electrosurgery and reduces surgery time, as cutting and coagulation are performed simultaneously.
Forceps and graspers:
There is a wide range of products with the following characteristics in various combinations: sharp or blunt, different lengths, different tips, different angles of the tips, with or without bipolar or monopolar coagulation. A bipolar blunt forceps with good gripping properties is very suitable for regular preparation. While using a cutting instrument with the right hand, the bipolar forceps enable grasping and coagulation with the left hand without changing the instrument.
Various necessary laparoscopic instruments:
Suction and irrigation devices, needle holders, different clip applicators, retrieval bags, retractors, and linear cutting staplers.
Instruments for robotic surgery:
All instruments mentioned above, such as graspers, scissors, retractors, and needle holders, are also available for robotic-assisted technology. The instruments have multiple joints and allow more degrees of freedom, similar to the mobility of a hand. Special tools such as linear cutting staplers are introduced into the abdominal cavity via separate trocars comparable to conventional laparoscopy and operated by the surgeon's assistant.
Hemostasis in Laparoscopic Surgery:
Because bleeding is minimized during laparoscopy, a good view of the operative field is achieved. Minor venous bleeding is limited by the working pressure, which may be raised up to 20 mmHg if significant venous bleeding is encountered to enable targeted coagulation. Hemostasis is performed depending on the severity with electrocoagulation, clips, or vascular sutures. The working pressure does not limit arterial bleeding. If major arterial bleeding persists despite all efforts, laparotomy and open surgery for hemostasis are necessary.
Electrocoagulation:
Bipolar electrocoagulation is safer and should be used exclusively. Monopolar electrocoagulation harbors incalculable risks due to instrument defects and fault currents.
Application of clips:
Standard titanium clips are available in multi-fire clip appliers for easy use without changing the instrument. Polymer clips with locking mechanisms are also available, enabling the transsection of large vessels such as the renal vein or artery.
Linear cutting stapler:
Linear cutting staplers are available for the transsection of large vessels or bowels. They are considerably more expensive than suture ligation or polymer clips with locking mechanisms, but they are more straightforward, safer, and faster.
Hemostats:
Hemostatic wound dressings or gels are used for hemostasis of larger surfaces, e.g., after partial nephrectomy.
Complications of Laparoscopy and Robotic Surgery
Comparison between laparoscopy and open surgery:
Laparoscopic surgery has a similar spectrum of side effects compared to open surgery. However, laparoscopy reduces complications due to the surgical approach (surgical site infections, pain, or abdominal hernia). In addition, laparoscopy minimizes bleeding complications, primarily due to the elevated abdominal pressure during the procedure, which minimizes venous bleeding and leads to an improved visualization of the surgical field. Overall, recovery is faster after laparoscopic surgery compared to open surgery.
Complications of the laparoscopic approach:
A variety of complications (bowel injury, vascular injury) may be caused by the establishment of pneumoperitoneum with the help of the Veress needle. The reasons are incorrect positions of the Veress cannula, adherent bowel loops to the abdominal wall, and injury due to the blind insertion of the first trocar. A safe open surgical technique for inserting the first trocar is highly recommended.
Complications caused by the pneumoperitoneum:
The increased intra-abdominal pressure (12–15 mmHg) leads to the following pathophysiological changes, which may trigger a decompensation if comorbidity is present:
Decreased venous backflow:
Low blood pressure, heart failure, venous thrombosis.
Increased airway pressures:
Pneumothorax, pneumomediastinum.
Hypercapnia:
The penetration of CO2 in tissue layers leads to emphysema and hypercapnia, which are usually compensated for by an increase in the respiratory minute volume.
Complications of electrocautery:
Complications of electrocautery are possible due to accidental coagulation of vital structures or technical defects (mainly due to monopolar electrocautery). Monopolar electrocautery harbors the risk of accidental coagulation due to faulty insulation of instrument shafts and unexpected electrical currents between the instrument and vital structures (e.g., ureter or bowel). Symptoms due to unnoticed thermal injury typically arise after 5–7 days. Bipolar coagulation offers a safe alternative to monopolar electrocautery and should be preferred.
| Perineal Approach | Index | Laser treatment in urology |
Index: 1–9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
References
Merseburger, A. S.; Herrmann, T. R. W.; Shariat, S. F.;
Kyriazis, I.; Nagele, U.; Traxer, O. & Liatsikos, E. N.
EAU
guidelines on robotic and single-site surgery in urology.
2013,
64, 277-291.
J. A. Smith, S. S. Howards, G. M. Preminger, and R. R. Dmochowski, Hinman’s Atlas of Urologic Surgery Revised Reprint. Elsevier, 2019.
 Deutsche Version: Laparoskopie in der Urologie
Deutsche Version: Laparoskopie in der Urologie
Urology-Textbook.com – Choose the Ad-Free, Professional Resource
This website is designed for physicians and medical professionals. It presents diseases of the genital organs through detailed text and images. Some content may not be suitable for children or sensitive readers. Many illustrations are available exclusively to Steady members. Are you a physician and interested in supporting this project? Join Steady to unlock full access to all images and enjoy an ad-free experience. Try it free for 7 days—no obligation.
New release: The first edition of the Urology Textbook as an e-book—ideal for offline reading and quick reference. With over 1300 pages and hundreds of illustrations, it’s the perfect companion for residents and medical students. After your 7-day trial has ended, you will receive a download link for your exclusive e-book.