You are here: Urology Textbook > Surgery (procedures) > Radical nephrectomy
Open Radical Nephrectomy: Surgical Steps and Complications
Indications for Radical Nephrectomy
Radical nephrectomy is the gold standard for treating large renal cell carcinomas if partial nephrectomy is not feasible. Radical nephrectomy removes the kidney with the perirenal fat and regional lymph nodes. The transperitoneal approach allows early control of the renal vessels, propagated by Robson (Robson et al., 1969). This dogma has never been studied prospectively, and many authors doubt its clinical significance (Mickisch et al., 2002). Accepted technical approaches of nephrectomy are transperitoneal, lumbar (flank incision), thoracoabdominal, laparoscopic, or retroperitoneoscopic, depending on the size and localization of the tumor.
 |
Contraindications for Radical Nephrectomy
Coagulation disorders. Do not perform radical nephrectomy if partial nephrectomy is technically possible, especially in patients with single kidneys, chronic kidney disease, bilateral renal cell cancer, or hereditary renal cell cancer. The other contraindications depend on the surgical risk due to the patient's comorbidity, the renal function of the contralateral kidney, and the surgical procedure's impact on the patient's life expectancy.
Surgical Technique
Preoperative patient preparation:
- Perioperative antibiotic prophylaxis if risk factors for surgical site infections are present.
- Perioperative indwelling catheter
- Perioperative gastric tube for a transperitoneal approach
- Consider epidural anesthesia
Transperitoneal approach:
- Supine position of the patients with mild hyperextension of the lumbar spine.
- Midline laparotomy or subcostal incision
- Divide and ligate the ligamentum teres hepatis to mobilize the liver. Incise the line of Toldt for medial reflection of the colon.
- On the left side: peritoneal incisions to mobilize the spleen.
- On the right side: Mobilize the duodenum from the vena cava (Kocher maneuver).
Flank approach:
Lateral decubitus position of the patient for a flank incision, the operation room table is flexed. The dissection is done between the 11th and 12th rib to spare the subcostal nerve. Open the renal fascia. Dissect bluntly the layer between the renal fascia and the psoas muscle. Dissect the peritoneum from the ventral portion of the renal fascia until you identify the renal vein. On the right side, mobilize the duodenum from the vena cava (Kocher maneuver).
Hilar preparation:
- On the left side, the division and ligation of the testicular or ovarian vein are necessary since the vein drains into the renal vein. The division is done below the inferior pole of the kidney. On the right side, the vein may be spared.
- Divide and ligate the ureter at the crossing with the iliac vessels. The ureter is followed to the renal hilum; the dissection should stay close to the vena cava (right side) or aorta (left side).
- Identify and dissect the renal vessels, and be aware of anomalies of the renal vessels. Visible lymph nodes are removed and sent for pathologic examination.
- Ligate the renal artery to stop renal blood flow.
- Divide renal vein between Overholt clamps; the vein should be closed with double (suture) ligation. After the division of the renal vein, the renal artery is now better visible and is divided with double (suture) ligation near the aorta. If significant atherosclerosis leads to a brittle artery, using 2–3 clips suitable for large vessels is wise.
Mobilization of the kidney:
Mobilize the kidney outside the renal fascia. The adrenal gland is separated from the adipose capsule and left in situ using blunt and sharp dissection. An adrenalectomy is necessary if large tumors of the upper kidney pole may invade the adrenal gland or if metastases are present in imaging.
Management of venous tumor thrombus:
Mobilize the complete kidney and divide or ligate the renal artery to stop renal blood flow. Small thrombi of the renal vein or vena cava do not require special measures: clamp the renal vein ostium with a Satinsky clamp, incise the renal vein ostium, and perform a thrombectomy first, followed by a nephrectomy. Close the defect of the vena cava with a double-row running suture.
Management of infrahepatic tumor thrombi:
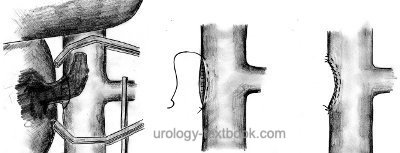
Carefully mobilize the vena cava; place vessel loops on both renal veins and the suprarenal and infrarenal vena cava. Clamp the vena cava proximal and distal to the tumor thrombus using Satinsky clamps. Clamp the contralateral renal vein and clip visible lumbar veins. The vena cava is opened (by circumcising the renal vein ostium), and the tumor thrombus is extracted [figure infrahepatic tumor thrombus resection]. Close the defect of the vena cava with a double-row running suture after nephrectomy. If the tumor thrombus invades the wall of the vena cava, resection of the caval wall and reconstruction of the IVC are necessary.
Management of intrahepatic and suprahepatic tumor thrombi:
It is possible to resect intrahepatic tumor thrombi while the suprahepatic vena cava inferior and the porta hepatis are clamped. If the tumor thrombus extends into the right atrium, the use of a cardiopulmonary bypass is inevitable. Consult visceral or cardiac surgeons before the surgery, depending on the cranial extension of the tumor thrombus.
Regional lymphadenectomy:
Lymphadenectomy is unnecessary for T1–2 tumors without suspicious lymph node enlargement since a large EORTC study demonstrated no survival benefit (Blom et al., 1999 and 2009). Some authors advocate, based on retrospective studies, a lymphadenectomy if advanced tumors or enlarged lymph nodes are present. The dissection template is from the crus of the diaphragm to the aortic bifurcation. Remove the para-aortic lymph nodes for left-sided tumors, the paracaval lymph nodes for right-sided tumors, and, regardless of the side, the interaortocaval lymph nodes (Capitanio et al., 2011).
Drains:
Drainage of the retroperitoneum is often performed, but it is probably unnecessary after an uncomplicated radical nephrectomy.
Postoperative Care after Radical Nephrectomy
General Measures:
- Early mobilization
- Intensive respiratory therapy
- Thrombosis prophylaxis
- Laboratory tests (hemoglobin, electrolytes, creatinine)
- Regular physical examination of the abdomen and incision wound.
Analgesia:
Analgesics with a combination of NSAIDs and opioids. Peridural anesthesia facilitates postoperative pain management.
Diet advancement:
Remove the nasogastric tube after surgery. Allow small sips of clear liquids after surgery. Increase clear liquids and allow yogurt or pudding on postoperative days 1 and 2. If the patient feels well, allow small amounts of solid food (appetite-driven) starting postoperative day 3.
Drains and Catheters:
Quick removal of the foley catheter after uneventful surgery for stable patients within 1–2 days, wound drainage (often unnecessary) for 1–2 days.
Complications of Radical Nephrectomy
Complications of radical nephrectomy for cT1--2 tumors can be seen in the table complications of radical and partial nephrectomy; the data is from randomized and retrospective studies.
| Complication | Radical nephrectomy | Partial nephrectomy |
| Significant hemorrhage | 1,1 % | 3,4 % |
| Hemorrhage <0,5 l | 96 % | 87 % |
| Urinoma | 0 % | 4 % |
| Reintervention | 2,4 % | 4,4 % |
| Mortality | 2 % | 1,6 % |
Risk of significant hemorrhage:
The risk of significant blood loss during radical nephrectomy is below 5%; the risk increases up to 35% if a tumor thrombus is present.
Injury of neighboring organs:
Liver injury, spleen injury (risk of splenectomy), paralytic ileus, bowel injury, peritonitis, pancreatic tail injury with fistula, pneumothorax, and chylous fistula due to injury of intestinal lymphatic vessels.
General complications:
Wound infection, heart attack, stroke, heart failure, thrombosis, pulmonary embolism, atelectasis, pneumonia, acute renal failure.
Mortality:
Mortality due to bleeding, cardiovascular diseases, arrhythmia, acute renal failure, and pulmonary embolism is around 1–2%, with advanced tumor stage being the leading risk factor.
| Simple Nephrectomy | Index | Laparoscopic nephrectomy |
Index: 1–9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
References
Blom u.a. 1999 BLOM, J. H. ; POPPEL, H. van ;
MARECHAL, J. M. ; JACQMIN, D. ; SYLVESTER, R. ;
SCHRODER, F. H. ; PRIJCK, L. de:
Radical nephrectomy with and without lymph node dissection:
preliminary results of the EORTC randomized phase III protocol 30881. EORTC
Genitourinary Group.
In: Eur Urol
36 (1999), Nr. 6, S. 570–5
Blom, Jan H M; van Poppel, Hein; Maréchal, Jean M;
Jacqmin, Didier; Schröder, Fritz H; de Prijck, Linda; Sylvester, Richard &
E. O. R. T. C. Genitourinary Tract Cancer Group
Radical nephrectomy
with and without lymph-node dissection: final results of European
Organization for Research and Treatment of Cancer (EORTC) randomized phase
3 trial 30881.
Eur Urol, 2009, 55, 28-34.
Capitanio, U.; Becker, F.; Blute, M. L.; Mulders, P.;
Patard, J.; Russo, P.; Studer, U. E. & Poppel, H. V.
Lymph node
dissection in renal cell carcinoma.
Eur Urol, 2011,
60, 1212-1220.
Corman u.a. 2000 CORMAN, J. M. ; PENSON,
D. F. ; HUR, K. ; KHURI, S. F. ; DALEY, J. ;
HENDERSON, W. ; KRIEGER, J. N.:
Comparison of complications after radical and partial nephrectomy:
results from the National Veterans Administration Surgical Quality
Improvement Program.
In: BJU Int
86 (2000), Nov, Nr. 7, S. 782–789
Mickisch 2002 MICKISCH, G. H.:
Principles of nephrectomy for malignant disease.
In: BJU Int
89 (2002), Nr. 5, S. 488–95
Poppel u.a. 2007 POPPEL, Hendrik V. ; POZZO,
Luigi D. ; ALBRECHT, Walter ; MATVEEV, Vsevolod ;
BONO, Aldo ; BORKOWSKI, Andrzej ; MARECHAL,
Jean-Marie ; KLOTZ, Laurence ; SKINNER, Eila ;
KEANE, Thomas ; CLAESSENS, Ilse ; SYLVESTER,
Richard ; RESEARCH for the European Organization for ;
CANCER (EORTC), Treatment of ; CANADA CLINICAL TRIALS GROUP
(NCIC CTG), National Cancer I. of ; (SWOG), Southwest Oncology G. ;
EASTERN COOPERATIVE ONCOLOGY GROUP (ECOG) the:
A prospective randomized EORTC intergroup phase 3 study comparing the
complications of elective nephron-sparing surgery and radical nephrectomy for
low-stage renal cell carcinoma.
In: Eur Urol
51 (2007), Jun, Nr. 6, S. 1606–1615
Robson u.a. 1969 ROBSON, C. J. ; CHURCHILL,
B. M. ; ANDERSON, W.:
The results of radical nephrectomy for renal cell carcinoma.
In: J Urol
101 (1969), Nr. 3, S. 297–301
Robson u.a. 2002 ROBSON, C. J. ; CHURCHILL,
B. M. ; ANDERSON, W.:
The results of radical nephrectomy for renal cell carcinoma. 1969.
In: J Urol
167 (2002), Nr. 2 Pt 2, S. 873–5; discussion 876–7
 Deutsche Version: Technik und Komplikationen der offen-chirurgischen Tumornephrektomie
Deutsche Version: Technik und Komplikationen der offen-chirurgischen Tumornephrektomie
Urology-Textbook.com – Choose the Ad-Free, Professional Resource
This website is designed for physicians and medical professionals. It presents diseases of the genital organs through detailed text and images. Some content may not be suitable for children or sensitive readers. Many illustrations are available exclusively to Steady members. Are you a physician and interested in supporting this project? Join Steady to unlock full access to all images and enjoy an ad-free experience. Try it free for 7 days—no obligation.
New release: The first edition of the Urology Textbook as an e-book—ideal for offline reading and quick reference. With over 1300 pages and hundreds of illustrations, it’s the perfect companion for residents and medical students. After your 7-day trial has ended, you will receive a download link for your exclusive e-book.