You are here: Urology Textbook > Prostate > Prostate cancer > Definition
Castration-Resistant Prostate Cancer (CRPC)
- Prostate cancer: Epidemiology and etiology
- Prostate cancer: Pathology
- Prostate cancer: Signs and symptoms
- Prostate cancer: Screening
- Prostate cancer: Staging
- Prostate cancer: Treatment options
- Prostate cancer: Active surveillance
- Prostate cancer: Prostatectomy
- Prostate cancer: Radiation therapy
- Prostate cancer: Brachytherapy
- Prostate cancer: TURP and experimental treatment options
- Prostate cancer: androgen deprivation therapy of advanced prostate cancer
- Prostate cancer: Treatment of castration-resistant prostate cancer
Guidelines and review literature: (EAU Guidelines Prostate Cancer) (S3-Leitlinie Prostatakarzinom) (Walsh-Campbell Urology).
Definition and Diagnosis of Castration-Resistant Prostate Cancer
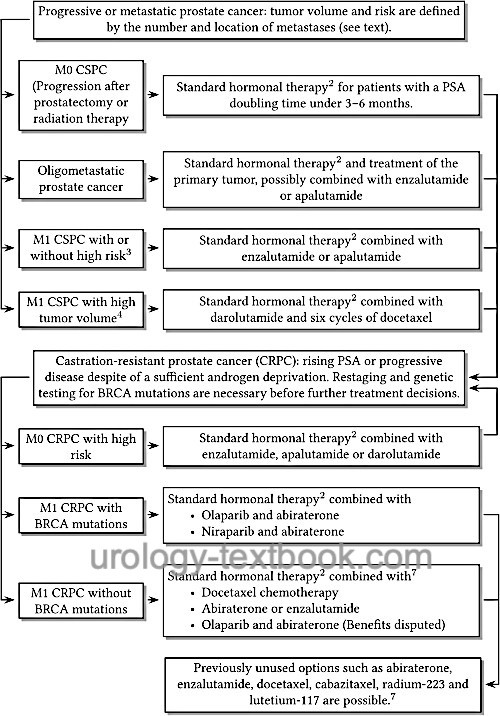
Castration-resistant prostate cancer (CRPC) is an advanced prostate carcinoma with biochemical (rising PSA concentration) or radiological progression despite sufficient androgen deprivation:
- M0 CRPC: Biochemical progression of prostate carcinoma under sufficient androgen deprivation and without evidence of metastases on imaging.
- M0 CRPC with high risk: M0 CRPC with a PSA doubling time of less than ten months.
- M1 CRPC: visible metastases and radiological progression despite sufficient androgen deprivation.
Mechanisms of Castration Resistance:
- Changes in the androgen receptor (gene amplification, overexpression)
- Overexpression of anti-apoptotic gene Bcl-2
- Activating of androgen-independent intracellular signal transduction for proliferation
- Co-factors of the androgen receptor which increase receptor activity
- Intracellular synthesis of testosterone from cholesterol
Since CRPC is still amenable to hormonal treatment, the old term hormone-refractory prostate cancer (HRPC) should no longer be used. The continuation of androgen deprivation is necessary despite castration resistance; it improves the prognosis and is given in combination with the below-mentioned treatment options, see also fig. androgen deprivation therapy of prostate cancer.
 |
Diagnosis:
To confirm the diagnosis of CRPC, the effectiveness of androgen deprivation should be monitored by serum testosterone.
Imaging:
CT (abdomen and thorax) and bone scintigraphy are the standard for detecting metastases. Modern imaging (PSMA-PET) leads to earlier detection of metastases, but the recommendations (and study results) mentioned below for CRPC M0 or M1 are based on the results of standard imaging. The additional benefit of PSMA-PET is unclear. Imaging should be repeated if the disease progresses.
Laboratory tests:
PSA concentration is measured every three months. Blood count, electrolytes, creatinine, liver enzymes, and AP are also important depending on symptoms or progression.
Genetic testing:
11% of CRPC patients have BRCA1/2 mutations in the tumor tissue. One-half are germline mutations, and the other half are somatic mutations (only in tumor tissue). Patients with BRCA mutations benefit particularly from treatment with olaparib (see below).
Treatment of Castration-Resistant Prostate Cancer
PARP Inhibitors
The poly-ADP-ribose polymerase (PARP) participates in cell DNA repair. In the pivotal study, the PARP inhibitor olaparib showed a significant response in patients with BRCA mutations and CRPC who were progressive on enzalutamide or abiraterone: prolonged progression-free survival (7 vs. 4 months) and prolonged survival (19 vs. 15 months). The main side effects were anemia and nausea (Bono et al., 2020). Olaparib (Lynparza) has been approved in Europe since 2020; requirements are progression of CRPC on newer androgen deprivation therapy and evidence of BRCA1/2 mutations. Rucaparib (Rubraca) is approved in the USA in patients with BRCA1/2 mutations who have been treated with androgen receptor-directed therapy and a taxane-based chemotherapy.
PARP inhibitor olaparib with abiraterone:
The combination of olaparib with abiraterone showed in the PROpel trial in patients with M1 CRPC and ineligible for chemotherapy (regardless of BRCA mutation status) prolonged progression-free survival (25 vs. 17 months) and overall survival (42 vs. 35 months) (Saad et al., 2023). However, the subgroup analysis showed only a slight advantage in overall survival in patients without BRCA mutations (HR 0.91, 40 vs. 38 months), while the difference in overall survival was overly clear in patients with BRCA mutations (HR 0.29). The combination of abiraterone, prednisolone, and olaparib has been approved as a first-line treatment for M1 CRPC in Europe without genetic testing since 2023. In the USA, approval was restricted to patients with BRCA mutations; there are concerns regarding the risk-benefit ratio in patients without BRCA mutations.
PARP inhibitor niraparib with abiraterone:
The MAGNITUDE trial (Chi et al., 2023) showed improved progression-free survival in patients with CRPC and BRCA mutations for whom chemotherapy was not indicated (20 vs. 11 months). Patients with HRR (homologous recombination repair) gene mutations also benefited (17 vs. 14 months). The analysis of overall survival shows no differences so far. The fixed combination Akeega (niraparib/abiraterone 100 mg/500 mg, two tablets daily) has been approved in Europe and the USA for first-line therapy in patients with CRPC and BRCA mutations since 2023.
PARP inhibitor talazoparib with enzalutamide:
The TALAPRO2 trial (Agarwal et al., 2023) showed improved progression-free survival in patients with CRPC treated with the combination talazoparib and enzalutamide (HR 0.63). An analysis of overall survival is not yet available. In the subgroup analysis, patients without BRCA mutations showed a significantly smaller advantage (HR 0.70), while the difference was clear in patients with BRCA mutations (HR 0.2). Talazoparib with enzalutamide has been approved in Europe since 2024 for the first-line treatment of patients with CRPC if chemotherapy is not clinically indicated. In Europe, the detection of a BRCA mutation is not mandatory for the treatment with talazoparib. In the USA, talazoparib is only approved for patients with BRCA mutations. The recommended dose for the combination is 0.5 mg talazoparib and 160 mg enzalutamide once daily.
Glucocorticoids in CRPC:
Glucocorticoids in CRPC can cause a weak response; they are an option in palliative care. Prednisolone (10 mg/d) must also be prescribed for all therapy regimens with abiraterone.
Abiraterone for M1 CRPC:
Abiraterone is a CYP-17 inhibitor and thus an inhibitor of testosterone biosynthesis [see section pharmacology/abiraterone for side effects and pharmacological details.]. Abiraterone is approved (also without olaparib) for the therapy of M1 CRPC before and after docetaxel chemotherapy. Dosage: 1000 mg 1-0-0 p.o. in combination with prednisolone 10 mg/d and standard androgen deprivation. Abiraterone prolonged survival in patients with M1 CRPC after docetaxel chemotherapy (14.8 months vs. 10.9 months with placebo) (Bono et al., 2011). Abiraterone is also beneficial before docetaxel chemotherapy: increased progression-free survival (16.5 vs. 8.3 months) and overall survival have been demonstrated (Ryan et al., 2013). Side effects are hepatotoxicity, edema, hypokalemia, hypertension by increasing the mineralocorticoids. Treatment is stopped if the disease progresses or the treatment is not tolerated.
Enzalutamide for M0 and M1 CRPC:
Enzalutamide is an androgen receptor antagonist with a high binding affinity. In addition, it prevents the translocation of activated androgen receptors to the nucleus. Enzalutamide has been approved for treating M0 CRPC with high risk and M1 CRPC before and after docetaxel chemotherapy. The dosage is 160 mg enzalutamide p.o. once daily in combination with standard androgen deprivation. The additional administration of prednisolone is possible but unnecessary. In phase III trials, enzalutamide significantly prolonged overall survival after docetaxel chemotherapy compared to placebo (18.4 vs. 13.6 months), with side effects comparable to the placebo group (Scher et al., 2012). In the PREVAIL trial, enzalutamide was effective before chemotherapy in castration-resistant prostate cancer (Beer et al., 2014). The PROSPER trial showed efficacy in M0 CRPC at high risk (PSA doubling time less than ten months): metastasis-free survival 37 vs. 15 months (Hussain et al., 2018). Important side effects include flushing, diarrhea, headache, and rarely epilepsy; see section pharmacology/enzalutamide for more details. Treatment is stopped if the disease progresses or the treatment is not tolerated.
Apalutamide for M0 CRPC:
Apalutamide is an androgen receptor antagonist that prevents the translocation of the activated androgen receptor into the nucleus and, thus, the transcription of target genes. Apalutamide has been approved for the treatment of high-risk M0 CRPC. The dosage is 240 mg (four 60 mg capsules) p.o. once daily in combination with standard androgen deprivation. Apalutamide was shown to prolong median metastasis-free survival (41 versus 16 months) in M0 CRPC with high risk (PSA doubling time less than ten months) (Smith et al., 2018a). Important side effects include skin rashes; see pharmacology/apalutamide for more details. Treatment is stopped if the disease progresses or the treatment is not tolerated.
Darolutamide for M0 CRPC:
Darolutamide is an androgen receptor antagonist that prevents translocation of the activated androgen receptor into the nucleus, thereby preventing transcription of target genes. Darolutamide has been approved for the treatment of high-risk M0 CRPC. The dosage is 600 mg darolutamide (two 300 mg capsules) p.o. in the morning and evening with meals, a daily dose of 1200 mg. In the pivotal trial (ARAMIS), treatment with darolutamide combined with classical antiandrogenic therapy improved metastasis-free survival (40 months vs. 18 months with placebo and androgen deprivation therapy), and overall survival was also better on trend (Fizazi et al., 2019). See pharmacology/darolutamide for more details.
Chemotherapy for Castration-Resistant Prostate Cancer
Docetaxel: First-Line Chemotherapy of Castration-Resistant Prostate Cancer
In terms of overall survival time, chemotherapy offers only a moderate improvement. Chemotherapy, however, leads to an improvement in pain associated with advanced disease. The indication for chemotherapy in castration-resistant prostate cancer is symptomatic disease (pain) with a rapid biochemical progression (PSA doubling time of less than three months). Chemotherapy should be given preference over androgen receptor antagonists in the following situations: low efficacy of androgen deprivation (progression after less than 12 months), presence of visceral metastases, and Gleason score ≥8.
The most effective treatment schedule is docetaxel 75 mg/m2 every three weeks (21-day schedule) in combination with prednisolone. This improves overall survival by three months (19.2 vs. 16.3) compared to mitoxantrone chemotherapy. The benefit is especially proven for patients with a good PSA response and Karnofsky index (Berthold et al., 2008). For pharmacology and side effects of docetaxel see section chemotherapy/docetaxel.
Cabazitaxel: Second-Line Chemotherapy of Castration-Resistant Prostate Cancer
Cabazitaxel, a taxane, is an effective chemotherapeutic agent for patients with CRPC in progression under or after chemotherapy with docetaxel. Overall survival was 15.1 vs. 12.7 months in phase III compared with mitoxantrone chemotherapy (Bono et al., 2010). Dosage: cabazitaxel 25 mg/m2 IV every three weeks with prednisolone 10 mg/d.
Prevention of Osseous Complications in Castration-Resistant Prostate Cancer
30–50% of patients with advanced prostate cancer suffer from pathological fractures due to bone metastases. The regular administration of a bisphosphonate (zoledronic acid) or a RANK ligand inhibitor (denosumab) inhibits osteoclasts and reduces morbidity from bone metastases.
Zoledronic acid:
Zoledronic acid reduced skeletal complications in Phase III trials: 33% skeletal-related events (SRE) with zoledronic acid vs. 44% SRE in the placebo group (Saad et al 2004). Serious complications include renal side effects and osteonecrosis of the jaw (2–5%). Dosage: 4 mg zoledronic acid IV every four weeks. Please see section pharmacology/zoledronic acid for pharmacological details.
Denosumab:
Denosumab is a human monoclonal antibody that interacts with RANKL and RANK and inhibits osteoclasts. Denosumab has been tested in a phase III trial advantageous compared to zoledronic acid: 20.7 months vs. 17.1 months until the onset of skeletal complications. Denosumab is administered subcutaneously; the dosage is 120 mg every four weeks. The risk of jaw osteonecrosis is comparable to zoledronic acid (Fizazi et al., 2011). To prevent osteoporosis, the dosage of denosumab is 60 mg s.c. every six months. Please see the section pharmacology/denosumab for pharmacological details.
Radiotherapy of Prostate Cancer Bone Metastases
Irradiation of Painful Lesions:
Palliative percutaneous radiotherapy is the treatment of choice for a single or few well-localized painful bone metastases. The dosage is around 30 Gy, which is administered in 10 fractions. A single fraction with a dose of 8 Gy is also possible.
Intravenous radionuclides for painful bone metastases:
Intravenous radionuclides are a treatment possibility for disseminated painful bone metastases. 223Ra (radium-223) is a modern radiopharmaceutical with alpha radiation, which was approved in 2013 by the FDA. Radium-223 is preferentially absorbed by bone (and thus in bone metastases) because of its chemical similarity to calcium. Due to the low range of an alpha emitter, only minor side effects on the bone marrow and other tissues have to be expected. The half-life of radium-223 is 11 days. In the pivotal study, patients with osseous metastatic castration-resistant prostate cancer and without visceral metastases were treated with six 223Ra injections every four weeks versus placebo (Parker et al., 2013). Significant differences were observed in survival time (15 vs. 11 months) and time to first skeletal complication of bone metastases (16 vs. 10 months). The rate of side effects was low: mild nausea and diarrhea. The treatment group had less bone pain (10 vs. 16%), anemia (8 vs. 9%) or spinal cord compression (4 vs. 5%).
A new therapeutic approach is possible with 117Lu-PSMA ligands (lutetium-117), which specifically bind to PSMA-expressing prostate carcinoma cells. Metastases should pick up sufficient tracer in diagnostic PSMA-PET. In the phase-3 trial, lutetium-117 showed prolonged progression-free survival (8.7 vs. 3.4 months) and overall survival (15 vs. 11 months) in heavily pretreated patients (Sartor et al., 2021). A complete remission was achieved in 9% of patients. The most common side effects were fatigue, nausea, dry mouth, worsening of renal function, and anemia. Lu-PSMA (Pluvicto) was approved in Europe and the USA in 2022 for PSMA-positive M1 CRPC treated with androgen receptor pathway inhibition and taxane-based chemotherapy.
Therapy of hydronephrosis:
Due to local progression or lymph node metastases, there is a possibility of obstructive uropathy of the upper urinary tract. Treatment with ureteral stents has a high failure rate in advanced disease with infiltration of the trigonum (bleeding, irritation, obstruction, and infection) necessitating percutaneous nephrostomy (DGU, 2009).
Future prospects in the treatment of advanced prostate carcinoma:
Many new agents are currently tested in clinical trials:
- PARP inhibitors: Rucaparib.
- Cabozantinib: is a tyrosine kinase inhibitor with activity against MET and VEGFR. The substance is currently being tested in several combinations.
- Immune checkpoint inhibition: ipilimumab or pembrolizumab have demonstrated antitumor activity in several phase I/II trials, but no survival benefit has been demonstrated in clinical trials to date.
- CDK4/6 inhibitors: Inhibitors of cyclin-dependent kinases (ribociclib, abemaciclib) are being tested in combination with chemotherapy or newer androgen deprivation therapy.
- PI3K/AKT signaling pathway: important mechanism of castration resistance, several substances (Ipatasertib, Capivasertib) with influence on the PI3K/AKT signaling pathway are in clinical trials.
- Androgen receptor signaling pathway: Further androgen synthesis inhibitors and androgen receptor degraders are being developed.
- Specific immune system activation: against prostate cancer proteins, several mechanisms are being tested: BiTE (bispecific T-cell engager) or CAR (chimeric antigen receptor) T-cells.
Follow-up Care for Prostate Cancer
Follow-up After Local Treatment
Follow-up care evaluates treatment side effects (micturition, sexuality) and measures the PSA concentration. Digital rectal examination is unnecessary if biochemical progress is ruled out. Imaging with PMSA-PET is only useful if local recurrence or disease progression is suspected and if imaging influences further therapy. For further details of the diagnosis and treatment of prostate cancer recurrence, see corresponding sections of radical prostatectomy or radiation therapy.
The schedule of follow-up examinations has yet to be systematically studied. The EAU guidelines recommend: three times in the first year (3, 6 and 12 months after therapy), every six months in the second and third year after therapy; thereafter, every year.
Follow-up of Advanced Prostate Cancer Treatment
Three and six months after starting androgen deprivation therapy, tolerability and clinical success (complaints, PSA concentration) are determined. Measuring the testosterone concentration is optional but mandatory if the treatment response with GnRH analogs is insufficient.
Further follow-up is planned individually. In patients with stage M0 and a good response to ADT, PSA monitoring and clinical investigations shoud be done every 3–6 months. Patients with clinical metastases require frequent examinations with additional blood count, creatinine, and AP measurements. Imaging should be performed individually based on symptoms, biochemical progression, and clinical consequences.
Prevention of Prostate Cancer
5α-reductase Inhibitors and Prostate Cancer Risk
In long-term randomized studies with 5α-reductase inhibitors to treat benign prostate hyperplasia, the incidence of prostate cancer was reduced (compared to placebo). For dutasteride, the reduced prostate cancer incidence was shown in the REDUCE trial. After 4 years of treatment with dutasteride 0.5 mg/d, the prostate cancer incidence in patients with increased risk was reduced to 20% (dutasteride) vs. 25% (placebo) (Andriole et al., 2010). The effect of 5α reductase inhibitors on prostate cancer mortality remains unclear, and the FDA recommended neither agent for the prevention of prostate cancer.
Selenium and Prostate Cancer Risk
Randomized trials and case-control studies could partly demonstrate a preventive effect of selenium on the development of prostate carcinoma. The meaningful SELECT study (see below) failed to show any preventive effect.
Antioxidants and Prostate Cancer Risk:
Both vitamin C and vitamin E have antioxidant properties, which are associated with reduced cancer risk. The Physicians Health Study II (randomized, n=14 600) could not prove any protective effect of vitamin C or E (Gaziano et al., 2009). This result is contrary to the results of SU.VI.MAX study (see below).
Inhibition of Cyclooxygenase (COX) and Prostate Cancer Risk
Several studies have demonstrated a lower prostate cancer risk in patients with regular intake of acetylsalicylic acid. The non-specific inhibition of cyclooxygenase leads to a problematic side effect profile. Prevention trials with COX-2 inhibitors are still pending.
Studies with the Combination of Several Substrates for the Prevention of Prostate Cancer
The data situation is contradictory; due to the SELECT study, substituting selenium or vitamin E is not considered helpful against prostate carcinoma.
SELECT trial:
Substitution with selenium, vitamin E, the combination of selenium with vitamin E or placebo (randomized, n=36 000 men). No differences between treatment groups and placebo (Lippman et al., 2009)
SU.VI.MAX study:
The daily substitution with vitamin C, vitamin E, beta-carotene, selenium and zinc reduces the incidence rate of PCA (randomized, n=5141, follow-up 8 years): prostate cancer risk halved (HR 0.52) in patients with a PSA below 3 ng/ml. However, the PSA concentration did not change due to the substitution and is therefore not a suitable biochemical marker for future prevention trials. In patients with pathological PSA levels, the reduction of prostate cancer risk was low (HR 0.88) (Meyer et al., 2005).
| Prostate cancer hormone treatment | Index | Prostate sarcoma |
Index: 1–9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
References
EAU Guidelines EAU - EANM - ESTRO - ESUR - SIOG Guidelines on Prostate Cancer, https://uroweb.org/guidelines/prostate-cancer/.
Andriole, G. L.; Bostwick, D. G.; Brawley, O. W.;
Gomella, L. G.; Marberger, M.; Montorsi, F.; Pettaway, C. A.; Tammela, T.
L.; Teloken, C.; Tindall, D. J.; Somerville, M. C.; Wilson, T. H.; Fowler,
I. L.; Rittmaster, R. S. & Group, R. E. D. U. C. E. S.
Effect of
dutasteride on the risk of prostate cancer.
N Engl J Med, 2010,
362, 1192-1202
Beer, T. M.; Armstrong, A. J.; Rathkopf, D. E.; Loriot, Y.; Sternberg, C. N.; Higano, C. S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; Davis, I. D.; de Bono, J. S.; Evans, C. P.; Fizazi, K.; Joshua, A. M.; Kim, C.-S.; Kimura, G.; Mainwaring, P.; Mansbach, H.; Miller, K.; Noonberg, S. B.; Perabo, F.; Phung, D.; Saad, F.; Scher, H. I.; Taplin, M.-E.; Venner, P. M.; Tombal, B. und Investigators, P. R. E. V. A. I. L. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med, 2014, 371, 424-433.
D. R. Berthold, G. R. Pond, F. Soban, R. de Wit, M. Eisenberger, and I. F.
Tannock.
Docetaxel plus prednisone or mitoxantrone plus prednisone for
advanced prostate cancer: updated survival in the tax 327 study.
J Clin Oncol, 26 (2): 242–245, Jan 2008.
URL https://dx.doi.org/10.1200/JCO.2007.12.4008.
K. N. Chi, et al., “Niraparib and Abiraterone Acetate for Metastatic Castration-Resistant Prostate Cancer.,” J Clin Oncol., vol. 41, no. 18, pp. 3339–3351, 2023.
De Bono, J. S.; Oudard, S.; Ozguroglu, M.; Hansen, S.;
Machiels, J.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M. J.; Shen,
L.; Roessner, M.; Gupta, S.; Sartor, A. O. & Investigators, T. R. O. P. I.
C.
Prednisone plus cabazitaxel or mitoxantrone for metastatic
castration-resistant prostate cancer progressing after docetaxel
treatment: a randomised open-label trial.
Lancet, 2010,
376, 1147-1154
De Bono, J. S.; Logothetis, C. J.; Molina, A.; Fizazi,
K.; North, S.; Chu, L.; Chi, K. N.; Jones, R. J.; Goodman, O. B.; Saad,
F.; Staffurth, J. N.; Mainwaring, P.; Harland, S.; Flaig, T. W.; Hutson,
T. E.; Cheng, T.; Patterson, H.; Hainsworth, J. D.; Ryan, C. J.;
Sternberg, C. N.; Ellard, S. L.; Fléchon, A.; Saleh, M.; Scholz, M.;
Efstathiou, E.; Zivi, A.; Bianchini, D.; Loriot, Y.; Chieffo, N.; Kheoh,
T.; Haqq, C. M.; Scher, H. I. & Investigators, C. O. U. A.
Abiraterone
and increased survival in metastatic prostate cancer.
N Engl J Med, 2011,
364, 1995-2005
Finlay, I. G.; Mason, M. D. & Shelley, M.
Radioisotopes
for the palliation of metastatic bone cancer: a systematic review.
Lancet
Oncol, 2005, 6, 392-400.
Fizazi, K.; Carducci, M.; Smith, M.; Damião, R.; Brown,
J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; Jiang, Q.;
Tadros, S.; Dansey, R. & Goessl, C.
Denosumab versus zoledronic
acid for treatment of bone metastases in men with castration-resistant
prostate cancer: a randomised, double-blind study.
Lancet, 2011,
377, 813-822
Gaziano, J. M.; Glynn, R. J.; Christen, W. G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J. E.; Sesso, H. D. &
Buring, J. E.
Vitamins E and C in the prevention of prostate and total
cancer in men: the Physicians' Health Study II randomized controlled trial.
JAMA,
2009, 301, 52–62
Goldfarb u.a. 2006 GOLDFARB, C. R. ;
SRIVASTAVA, Neil C. ; GROTAS, Aaron B. ; ONGSENG,
Fukiat ; NAGLER, Harris M.:
Radionuclide imaging in urology.
In: Urol Clin North Am
33 (2006), Aug, Nr. 3, S. 319–328
Lippman, S. M.; Klein, E. A.; Goodman, P. J.; Lucia, M.
S.; Thompson, I. M.; Ford, L. G.; Parnes, H. L.; Minasian, L. M.; Gaziano,
J. M.; Hartline, J. A.; Parsons, J. K.; Bearden, J. D.; Crawford, E. D.;
Goodman, G. E.; Claudio, J.; Winquist, E.; Cook, E. D.; Karp, D. D.;
Walther, P.; Lieber, M. M.; Kristal, A. R.; Darke, A. K.; Arnold, K. B.;
Ganz, P. A.; Santella, R. M.; Albanes, D.; Taylor, P. R.; Probstfield, J.
L.; Jagpal, T. J.; Crowley, J. J.; Meyskens, F. L.; Baker, L. H. &
Coltman, C. A.
Effect of selenium and vitamin E on risk of prostate
cancer and other cancers: the Selenium and Vitamin E Cancer Prevention
Trial (SELECT).
JAMA, 2009, 301, 39–51
Kantoff, P. W.; Higano, C. S.; Shore, N. D.; Berger, E.
R.; Small, E. J.; Penson, D. F.; Redfern, C. H.; Ferrari, A. C.; Dreicer,
R.; Sims, R. B.; Xu, Y.; Frohlich, M. W.; Schellhammer, P. F. &
Investigators, I. M. P. A. C. T. S.
Sipuleucel-T immunotherapy for
castration-resistant prostate cancer.
N Engl J Med, 2010,
363, 411-422
Meyer u.a. 2005 MEYER, F. ; GALAN, P. ;
DOUVILLE, P. ; BAIRATI, I. ; KEGLE, P. ;
BERTRAIS, S. ; ESTAQUIO, C. ; HERCBERG, S.:
Antioxidant vitamin and mineral supplementation and prostate cancer
prevention in the SU.VI.MAX trial.
In: Int J Cancer
116 (2005), Nr. 2, S. 182–6
Miller, K.
[Castration resistant prostate cancer 2011].
Aktuelle Urol, 2011, 42, 95-102
Ryan, C. J.; Smith, M. R.; de Bono, J. S.; Molina, A.;
Logothetis, C. J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.
M.; Ng, S.; Carles, J.; Mulders, P. F. A.; Basch, E.; Small, E. J.; Saad,
F.; Schrijvers, D.; Poppel, H. V.; Mukherjee, S. D.; Suttmann, H.;
Gerritsen, W. R.; Flaig, T. W.; George, D. J.; Yu, E. Y.; Efstathiou, E.;
Pantuck, A.; Winquist, E.; Higano, C. S.; Taplin, M.; Park, Y.; Kheoh, T.;
Griffin, T.; Scher, H. I.; Rathkopf, D. E. & Investigators, C. O. U. A.
Abiraterone
in metastatic prostate cancer without previous chemotherapy.
N Engl
J Med, 2013, 368, 138-148.
Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S. I.;
O'Sullivan, J. M.; Fosså, S. D.; Chodacki, A.; Wiechno, P.; Logue, J.;
Seke, M.; Widmark, A.; Johannessen, D. C.; Hoskin, P.; Bottomley, D.;
James, N. D.; Solberg, A.; Syndikus, I.; Kliment, J.; Wedel, S.; Boehmer,
S.; Dall'Oglio, M.; Franzén, L.; Coleman, R.; Vogelzang, N. J.;
O'Bryan-Tear, C. G.; Staudacher, K.; Garcia-Vargas, J.; Shan, M.; Bruland,
Ø. S.; Sartor, O. & Investigators, A. L. S. Y. M. P. C. A.
Alpha emitter radium-223 and survival in metastatic prostate cancer.
New Engl J Med 2013,
369, 213-223
O. Sartor et al., “Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer.,” NEJM, vol. 385, no. 12, pp. 1091–1103, 2021.
Leitlinienprogramm Onkologie (DGU, Deutsche Krebsgesellschaft, Deutsche Krebshilfe): Interdisziplinäre Leitlinie der Qualität S3 zur Früherkennung, Diagnose und Therapie der verschiedenen Stadien des Prostatakarzinoms https://www.leitlinienprogramm-onkologie.de/leitlinien/prostatakarzinom/
Wein, A. J.; Kavoussi, L. R.; Partin, A. P. & Peters, C. A.
Campbell-Walsh Urology
. Elsevier, 2015. ISBN 978-1455775675.
F. Saad et al., “PROpel: Phase III trial of olaparib (ola) and abiraterone (abi) versus placebo (pbo) and abi as first-line (1L) therapy for patients (pts) with metastatic castration-resistant prostate cancer (mCRPC).,” Journal of Clinical Oncology, vol. 40, no. 6suppl, p. 11, 2022.
Saad, F.; Gleason, D. M.; Murray, R.; Tchekmedyian, S.;
Venner, P.; Lacombe, L.; Chin, J. L.; Vinholes, J. J.; Goas, J. A. &
Zheng, M.
Long-term efficacy of zoledronic acid for the prevention of
skeletal complications in patients with metastatic hormone-refractory
prostate cancer
J Natl Cancer Inst, 2004, 96,
879-82
Scher, H. I.; Fizazi, K.; Saad, F.; Taplin, M.;
Sternberg, C. N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K. N.; Shore,
N. D.; Armstrong, A. J.; Flaig, T. W.; Fléchon, A.; Mainwaring, P.;
Fleming, M.; Hainsworth, J. D.; Hirmand, M.; Selby, B.; Seely, L.; de
Bono, J. S. & Investigators, A. F. F. I. R. M.
Increased survival
with enzalutamide in prostate cancer after chemotherapy.
N Engl J
Med, 2012, 367, 1187-1197.
 Deutsche Version: Prostatakarzinom
Deutsche Version: Prostatakarzinom
Urology-Textbook.com – Choose the Ad-Free, Professional Resource
This website is designed for physicians and medical professionals. It presents diseases of the genital organs through detailed text and images. Some content may not be suitable for children or sensitive readers. Many illustrations are available exclusively to Steady members. Are you a physician and interested in supporting this project? Join Steady to unlock full access to all images and enjoy an ad-free experience. Try it free for 7 days—no obligation.
New release: The first edition of the Urology Textbook as an e-book—ideal for offline reading and quick reference. With over 1300 pages and hundreds of illustrations, it’s the perfect companion for residents and medical students. After your 7-day trial has ended, you will receive a download link for your exclusive e-book.