You are here: Urology Textbook > Drugs in Urology > Androgen Deprivation Therapy
Androgen Deprivation Therapy: Drugs and Adverse Effects
Androgen deprivation therapy (ADT) is a treatment option for advanced prostate cancer in the following situations:
- Metastatic prostate cancer
- Biochemical progression of prostate cancer with no detectable metastases and a PSA doubling time below three months
- Adjuvant therapy of advanced prostate cancer after radical prostatectomy or radiation therapy
- Conservative treatment of locally advanced prostate cancer with LUTS
- Lowering prostate volume before brachytherapy
Classification of Drugs for Androgen Deprivation Therapy
- Classic androgen receptor antagonists: nonsteroidal drugs are bicalutamide and flutamide. Cyproterone acetate is a classic steroidal antiandrogen with a progestational effect, which causes additional central inhibition of the testosterone concentration.
- GnRH agonists: Buserelin, Goserelin, Histrelin, Leuprorelin, and Triptorelin
- GnRH antagonists: degarelix and relugolix
- Estrogens: Fosfestrol
- Androgen synthesis inhibitors: Abiraterone.
- Modern androgen receptor antagonists: Enzalutamide, Apalutamide, Darolutamide.
Principles of Androgen Deprivation Therapy
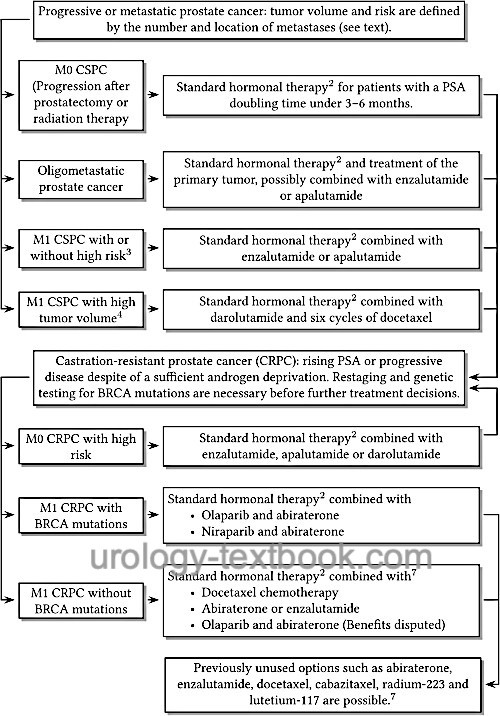
The individual therapy options are applied sequentially or in combination depending on disease progression and response [fig. androgen deprivation therapy of prostate cancer]. Please see also the section on metastatic prostate cancer for details.
 |
General Adverse Effects of Androgen Deprivation Therapy
Androgen Deprivation Therapy (ADT) leads to significant side effects, especially in long-term use. Androgen receptor antagonists do not lower the testosterone concentration and cause fewer side effects (except gynecomastia) compared to orchiectomy or GnRH modulators. General measures for the prevention of the side effects listed below are increasing physical activity, striving for a normal body weight, eating a vitamin-rich, healthy diet, and not smoking (Nguyen et al., 2015).
Hot flashes:
Hot flashes are very common; treatment options are (off-label use) Clonidine, Cyproteronacetat, Venlafaxine, or Paroxetine (Loprinzi et al., 2004).
Erectile dysfunction and loss of libido:
ADT leads to severe erectile dysfunction (over 80%) and loss of libido (over 95%). Effective treatment of libido loss is not possible, but the psychological burden of sexual dysfunction is low. Therapeutic options against erectile dysfunction (PDE5 inhibitors or SKAT) are not often requested. Long-term ADT leads to reductions in both penile and testicular size.
Gynecomastia and mastodynia:
Gynecomastia is a very common side effect of androgen receptor antagonists. When bicalutamide 150 mg is administered alone, the risk for gynecomastia is well over 70% after one year. The prophylactic single irradiation with 12–18 Gy of the mammary glands before treatment significantly lowers the risk to 30–50 %. The risk of gynecomastia for combined androgen deprivation therapy is lower and amounts to about 20%. Gynecomastia is rare if GnRH modulators are used alone.
The prophylactic administration of tamoxifen (off-label) is more potent than the irradiation to reduce the frequency of gynecomastia (10% vs. 30–50%). The dosage of tamoxifen is 20 mg/d. The effect of tamoxifen does not appear to affect androgen deprivation therapy and the biological behavior of prostate cancer (Fradet et al., 2007). Another therapy option is subcutaneous mastectomy.
Cognitive function and mood disorders:
Cognitive function is disturbed by ADT. Furthermore, decreased libido, fatigue, and episodes of depression (relative risk RR of 8) are more common.
Metabolic changes:
Androgen deprivation leads to a loss of muscle mass and increase in body fat, after a year the body weight has increased on average 2–4%. The risk of metabolic syndrome and type II diabetes is also significantly increased.
Cardiovascular risks:
Androgen deprivation leads to an increase in cardiovascular diseases (the relative risk in brackets): CHD (RR 1.2), myocardial infarction (RR 1.1), life-threatening cardiac arrhythmias, or sudden cardiac death (RR 1.16). Cardiovascular mortality increases by 1–6% compared to the placebo group, depending on the study and follow-up period (Levine et al., 2010).
Osteoporosis and fractures:
Osteoporosis is a common complication of long-term ADT with GnRH modulators or castration (Diamond et al., 2004b). The administration of androgen receptor blockers (e.g., Bicalutamide) is not associated with osteoporosis (SmithMR et al, 2004). The fracture rate among men surviving at least 5 years is 19% with ADT versus 13% without ADT. All patients with a long-term prognosis should receive prophylactic vitamin D (800–1000 IU) and calcium (1000 mg). Additional treatment options for osteoporosis with increased fracture risk include bisphosphonates or denosumab.
Anemia:
Normochromic normocytic anemia develops in patients with long-term ADT, which responds well to erythropoietin (Choo et al., 2005).
| GnRH agonists | Index | Flutamide |
Index: 1–9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
References
Anderson 2003 ANDERSON, J.:
The role of antiandrogen monotherapy in the treatment of prostate
cancer.
In: BJU Int
91 (2003), Nr. 5, S. 455–61.
Choo u.a. 2005 CHOO, R. ; CHANDER, S. ;
DANJOUX, C. ; MORTON, G. ; PEARCE, A. ;
DEBOER, G. ; SZUMACHER, E. ; LOBLAW, A. ;
CHEUNG, P. ; WOO, T.:
How are hemoglobin levels affected by androgen deprivation in
non-metastatic prostate cancer patients?
In: Can J Urol
12 (2005), Nr. 1, S. 2547–52.
Diamond u.a. 2004 DIAMOND, T. H. ; HIGANO,
C. S. ; SMITH, M. R. ; GUISE, T. A. ; SINGER,
F. R.:
Osteoporosis in men with prostate carcinoma receiving
androgen-deprivation therapy: recommendations for diagnosis and therapies.
In: Cancer
100 (2004), Nr. 5, S. 892–9.
Fradet u.a. 2007 FRADET, Yves ; EGERDIE,
Blair ; ANDERSEN, Morten ; TAMMELA, Teuvo L J. ;
NACHABE, Mahmoud ; ARMSTRONG, Jon ; MORRIS,
Thomas ; NAVANI, Sunil:
Tamoxifen as prophylaxis for prevention of gynaecomastia and breast
pain associated with bicalutamide 150 mg monotherapy in patients with
prostate cancer: a randomised, placebo-controlled, dose-response study.
In: Eur Urol
52 (2007), Jul, Nr. 1, S. 106–114.
Levine, Glenn N; D'Amico, Anthony V; Berger, Peter;
Clark, Peter E; Eckel, Robert H; Keating, Nancy L; Milani, Richard V;
Sagalowsky, Arthur I; Smith, Matthew R; Zakai, Neil; on Clinical
Cardiology, American Heart Association Council; on Epidemiology, Council;
Prevention, the American Cancer Society & the American Urological
Association
Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association:
endorsed by the American Society for Radiation Oncology.
CA Cancer J Clin, 2010, 60, 194-201
Loblaw u.a. 2004 LOBLAW, D. A. ; MENDELSON,
D. S. ; TALCOTT, J. A. ; VIRGO, K. S. ;
SOMERFIELD, M. R. ; BEN-JOSEF, E. ; MIDDLETON,
R. ; PORTERFIELD, H. ; SHARP, S. A. ; SMITH,
T. J. ; TAPLIN, M. E. ; VOGELZANG, N. J. ; WADE,
Jr. ; BENNETT, C. L. ; SCHER, H. I.:
American Society of Clinical Oncology recommendations for the initial
hormonal management of androgen-sensitive metastatic, recurrent, or
progressive prostate cancer.
In: J Clin Oncol
22 (2004), Nr. 14, S. 2927–41.
Loprinzi u.a. 2004 LOPRINZI, C. L. ; BARTON,
D. L. ; CARPENTER, L. A. ; SLOAN, J. A. ; NOVOTNY,
P. J. ; GETTMAN, M. T. ; CHRISTENSEN, B. J.:
Pilot evaluation of paroxetine for treating hot flashes in men.
In: Mayo Clin Proc
79 (2004), Nr. 10, S. 1247–51.
Montgomery u.a. 2005 MONTGOMERY, B. S. ;
BORWELL, J. P. ; HIGGINS, D. M.:
Does needle size matter? Patient experience of luteinising
hormone-releasing hormone analogue injection.
In: Prostate Cancer Prostatic Dis
8 (2005), Nr. 1, S. 66–8.
Miyamoto u.a. 2004 MIYAMOTO, H. ; MESSING,
E. M. ; CHANG, C.:
Androgen deprivation therapy for prostate cancer: current status and
future prospects.
In: Prostate
61 (2004), Nr. 4, S. 332–53.
Perdona u.a. 2005 PERDONA, S. ; AUTORINO, R. ;
DE PLACIDO, S. ; D’ARMIENTO, M. ; GALLO, A. ;
DAMIANO, R. ; PINGITORE, D. ; GALLO, L. ;
DE SIO, M. ; BIANCO, A. R. ; DI LORENZO, G.:
Efficacy of tamoxifen and radiotherapy for prevention and treatment
of gynaecomastia and breast pain caused by bicalutamide in prostate cancer: a
randomised controlled trial.
In: Lancet Oncol
6 (2005), Nr. 5, S. 295–300.
Sieber u.a. 2004 SIEBER, P. R. ; KEILLER,
D. L. ; KAHNOSKI, R. J. ; GALLO, J. ; MCFADDEN,
S.:
Bicalutamide 150 mg maintains bone mineral density during monotherapy
for localized or locally advanced prostate cancer.
In: J Urol
171 (2004), Nr. 6 Pt 1, S. 2272–6, quiz 2435
Smith u.a. 2003 SMITH, M. R. ; EASTHAM, J. ;
GLEASON, D. M. ; SHASHA, D. ; TCHEKMEDYIAN, S. ;
ZINNER, N.:
Randomized controlled trial of zoledronat to prevent bone loss
in men receiving androgen deprivation therapy for nonmetastatic prostate
cancer.
In: J Urol
169 (2003), Nr. 6, S. 2008–12.
Smith u.a. 2004a SMITH, M. R. ;
FALLON, M. A. ; LEE, H. ; FINKELSTEIN, J. S.:
Raloxifene to prevent gonadotropin-releasing hormone agonist-induced
bone loss in men with prostate cancer: a randomized controlled trial.
In: J Clin Endocrinol Metab
89 (2004), Nr. 8, S. 3841–6.
Smith u.a. 2004b SMITH, M. R. ;
GOODE, M. ; ZIETMAN, A. L. ; MCGOVERN, F. J. ;
LEE, H. ; FINKELSTEIN, J. S.:
Bicalutamide monotherapy versus leuprolide monotherapy for prostate
cancer: effects on bone mineral density and body composition.
In: J Clin Oncol
22 (2004), Nr. 13, S. 2546–53.
Tyrrell u.a. 2004 TYRRELL, C. J. ; PAYNE, H. ;
TAMMELA, T. L. ; BAKKE, A. ; LODDING, P. ;
GOEDHALS, L. ; VAN ERPS, P. ; BOON, T. ;
VAN DE BEEK, C. ; ANDERSSON, S. O. ; MORRIS, T. ;
CARROLL, K.:
Prophylactic breast irradiation with a single dose of electron beam
radiotherapy (10 Gy) significantly reduces the incidence of
bicalutamide-induced gynecomastia.
In: Int J Radiat Oncol Biol Phys
60 (2004), Nr. 2, S. 476–83.
Widmark u.a. 2003 WIDMARK, A. ; FOSSA, S. D. ;
LUNDMO, P. ; DAMBER, J. E. ; VAAGE, S. ;
DAMBER, L. ; WIKLUND, F. ; KLEPP, O.:
Does prophylactic breast irradiation prevent antiandrogen-induced
gynecomastia? Evaluation of 253 patients in the randomized Scandinavian trial
SPCG-7/SFUO-3.
In: Urology
61 (2003), Nr. 1, S. 145–51.
 Deutsche Version: Antiandrogene Therapie
Deutsche Version: Antiandrogene Therapie
Urology-Textbook.com – Choose the Ad-Free, Professional Resource
This website is designed for physicians and medical professionals. It presents diseases of the genital organs through detailed text and images. Some content may not be suitable for children or sensitive readers. Many illustrations are available exclusively to Steady members. Are you a physician and interested in supporting this project? Join Steady to unlock full access to all images and enjoy an ad-free experience. Try it free for 7 days—no obligation.
New release: The first edition of the Urology Textbook as an e-book—ideal for offline reading and quick reference. With over 1300 pages and hundreds of illustrations, it’s the perfect companion for residents and medical students. After your 7-day trial has ended, you will receive a download link for your exclusive e-book.