You are here: Urology Textbook > Ureters > Retroperitoneal fibrosis
Retroperitoneal Fibrosis: Diagnosis and Treatment of Ormond Disease
Definition of Retroperitoneal Fibrosis
Ormond disease (retroperitoneal fibrosis) is a disease with an unknown etiology, leading to increasing fibrosis of the retroperitoneum and compression of ureters and retroperitoneal vessels (Vaglio et al., 2006).
 |
Epidemiology of Retroperitoneal Fibrosis
Prevalence 1–2/100.000, age of onset 40–60 years, male to female ratio = 2:1.
Causes (Etiology) of Retroperitoneal Fibrosis
Unknown Etiology
In 70% of patients with retroperitoneal fibrosis, the etiology of Ormond disease remains unclear. One assumed disease mechanism is an autoimmune process with periaortitis as the initial pathophysiological mechanism. Later, the inflammatory-fibrotic mass extends into the retroperitoneum and compresses the ureters. There is also evidence of a local manifestation of a systemic increased willingness to develop autoimmune diseases (see below association with fibrotic diseases).
Known Triggers
Drugs:
Ergot alkaloids, β-blocker, phenacetin, LSD, haloperidol, amphetamine, reserpine.
Infections:
Lymphangitis, chronic inflammatory bowel disease, gonorrhea, syphilis, urogenital tuberculosis, chronic urinary tract infection, sarcoidosis.
Association with fibrotic diseases:
Sclerosing mediastinitis, sclerosing cholangitis, orbital pseudotumor, Riedel's thyroiditis. In children association with systemic lupus erythematosus or juvenile rheumatoid arthritis.
Other risk factors:
Irradiation, retroperitoneal bleeding, pelvic surgery, Purpura Hennoch.
Pathology of Retroperitoneal Fibrosis
Retroperitoneal fibrosis leads to a substantial mass of connective tissue, which surrounds the large retroperitoneal vessels and may include the ureter. The retroperitoneal mass extends from the renal hilum to the sacral promontory in the craniocaudal direction. At the edges of the active fibrosis are signs of inflammation in the connective tissue (lymphocytes, plasma cells, histiocytes). In the center of the mass, inactive connective tissue is found without signs of inflammation.
Signs and Symptoms
- Flank pain: constant dull pain, starting in the flanks with radiating to the lower abdomen.
- Nonspecific symptoms such as weight loss, malaise, nausea, and vomiting may occur at the beginning of the inflammatory process. Lymphadenopathy is not typical.
- In untreated patients, symptoms of uremia may develop.
Diagnostic Workup of Retroperitoneal Fibrosis
Laboratory tests:
Elevated erythrocyte sedimentation rate (ESR) and CRP. Further necessary tests are creatinine, electrolytes, complete blood count, autoantibodies (ANA, ANCA), immunoelectrophoresis, and rheumatoid factor. Additional tests in men: PSA, AFP, HCG, LDH.
Ultrasound imaging:
Renal ultrasound imaging reliably identifies hydronephrosis. Reduced thickness of the kidney parenchyma is an sign of chronic renal failure. The retroperitoneal connective tissue mass around the aorta can be visualized in good conditions.
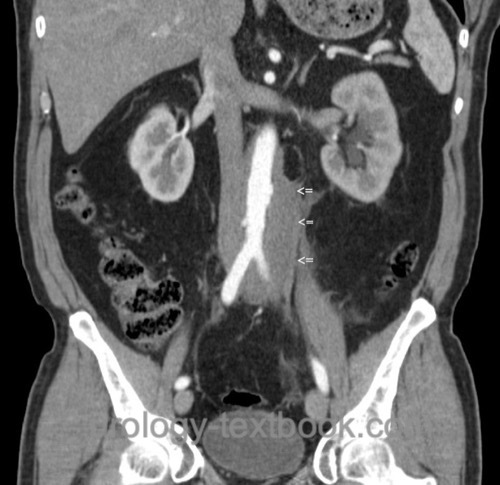
CT or MRI of the Abdomen:
Abdominal imaging reveals a flat mass surrounding the great vessels and the ureters with bilateral hydronephrosis. The renal function or damage can be estimated with the thickness of the renal parenchyma.
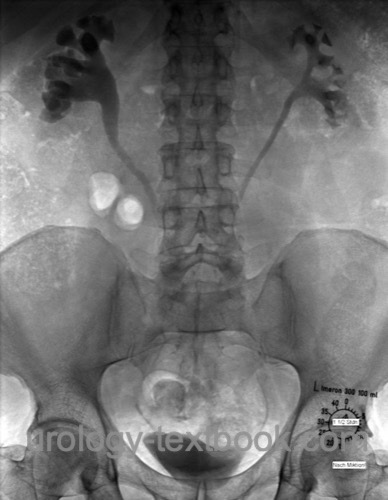
Intravenous urogram:
Intravenous urography reveals bilateral hydronephrosis and displacement of the proximal and mid-ureter to the middle. IVP is only necessary if cross-sectional imaging is not available.
 |
Retrograde pyelography:
Retrograde pyelography is indicated in patients with contraindications to contrast medium (e.g., severe chronic kidney disease) and before endoscopic therapy. Radiological signs are similar to intravenous urography; intrinsic ureteral obstructions are easily identified.
Renal Scintigraphy:
Perform renal scintigraphy to determine spit renal function and to objectify urinary obstruction at the onset and later in the course of the disease.
Percutaneous Biopsy:
Biopsy of the retroperitoneal mass should be done to exclude a malignant etiology, especially with suspected retroperitoneal lymphoma (pathological blood count, lymphadenopathy) or cancer in the history. With typical clinical and radiological criteria of retroperitoneal fibrosis, it is controversial whether a biopsy is necessary before medical treatment.
Differential Diagnosis of Retroperitoneal Fibrosis
The most common differential diagnosis is lymphoma; less common are carcinoid, multiple myeloma, pancreatic cancer, prostate cancer metastases, sarcoma, or urogenital tuberculosis.
Treatment of Retroperitoneal Fibrosis
Acute treatment of hydronephrosis
The initial treatment of hydronephrosis is retrograde pyelography and placement of DJ ureteral stents.
Medical Treatment of Retroperitoneal Fibrosis
Steroid therapy with prednisolone 1 mg/kgBW per day for two days, then reduction of the daily dose to 40 mg, 20 mg, 10 mg every two weeks, maintenance dose 5 mg per day for one year (Brandt et al., 2016). Immunosuppressants such as azathioprine and mycophenolic acid are added depending on the response and side effects. Treatment success has also been reported with tamoxifen. Steroid therapy is particularly effective in patients with active signs of inflammation (elevated ESR, leukocytosis).
Secondary retroperitoneal fibrosis:
Diagnose and treat possible causes; see etiology and known triggers.
Surgical Treatment of Retroperitoneal Fibrosis
The surgical treatment (ureterolysis and intraperitoneal transfer of the ureter) is indicated for failure of medical treatment or if diagnosis by biopsy is unclear. Nephrectomy is indicated for a nonfunctioning kidney.
Technique of the ureterolysis:
Preoperatively, new DJ stents are inserted. After a median laparotomy and incision of both paracolic gutters, the colon is mobilized medially on both sides to expose the retroperitoneum. Biopsies from the retroperitoneal mass are sent for frozen section examination. The ureters are freed from the retroperitoneal mass; the dissection is best done from distal to proximal. After complete mobilization of the ureters, they are relocated to the intraperitoneal cavity, and the peritoneum is closed again. The hiatus of the peritoneum for the ureters must be large enough to prevent iatrogenic ureteral compression. Omentum majus can be wrapped around the ureters to separate the retroperitoneal fibrosis safely. Alternatively, the ureters are left in the retroperitoneum lateral to the fibrotic mass.
Ureter reconstruction:
Depending on the manifestation of the disease and involvement of the ureter, the ureter must be replaced segmentally or completely. The following techniques are used: reimplantation with Boari flap, intestinal interposition, or autologous kidney transplantation, see section ureteral stricture.
Follow-up
Retroperitoneal fibrosis is a chronic relapsing disease, lifelong follow-up care is necessary (ESR, CRP, sonography of the kidneys and possibly CT/MRI every 1–2 years after discontinuation of immunosuppression).
| Ureter, anatomy | Index | Ureter, diseases |
Index: 1–9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
References
Vaglio u.a. 2006 VAGLIO, A. ; SALVARANI, C. ; BUZIO, C.: Retroperitoneal fibrosis.In: Lancet
367 (2006), Nr. 9506, S. 241–51
 Deutsche Version: Morbus Ormond: retroperitoneale Fibrose
Deutsche Version: Morbus Ormond: retroperitoneale Fibrose
Urology-Textbook.com – Choose the Ad-Free, Professional Resource
This website is designed for physicians and medical professionals. It presents diseases of the genital organs through detailed text and images. Some content may not be suitable for children or sensitive readers. Many illustrations are available exclusively to Steady members. Are you a physician and interested in supporting this project? Join Steady to unlock full access to all images and enjoy an ad-free experience. Try it free for 7 days—no obligation.
New release: The first edition of the Urology Textbook as an e-book—ideal for offline reading and quick reference. With over 1300 pages and hundreds of illustrations, it’s the perfect companion for residents and medical students. After your 7-day trial has ended, you will receive a download link for your exclusive e-book.