You are here: Urology Textbook > Bladder > Neurogenic Lower Urinary Tract Dysfunction
Neurogenic Lower Urinary Tract Dysfunction: Diagnosis and Treatment
Diagnosis of NLUTD
History:
Important are micturition symptoms, incontinence severity (pad usage), previous surgery, medications (e.g., alpha blocker, clonidine), neurological and urological diseases. Ask for symptoms of pelvic prolapse and bowel and sexual dysfunction.
Physical examination:
Urological and gynecological examination: evidence of stress incontinence? Short neurological examination: Sensitivity test for each (sacral) dermatome, muscle strength test, biceps tendon reflex, Achilles tendon reflex, patellar tendon reflex, Babinski sign, anal reflex, bulbo spongiosus reflex, cremaster reflex, anal reflex and sphincter tonus.
- Absent reflex response: acute damage to the first motor neuron (acute paraplegia) or damage to the peripheral nerves (herniated disc, nerve injury).
- Hyperreflexia: upper motor neuron disease (paraplegia, stroke), further signs of pyramidal tract damage are a positive Babinski sign.
Laboratory tests:
Urine sediment and urine culture, serum creatinine.
Ultrasound imaging:
Hydronephrosis? Thickness of the renal parenchyma? Residual urine? Bladder wall thickness?
Cystoscopy:
Perform cystoscopy to rule out differential diagnoses such as tumors, cystitis, or urinary bladder stones.
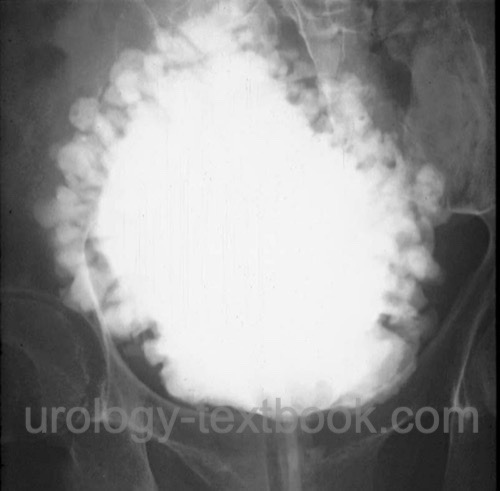 |
VCUG:
VCUG is usually performed during urodynamics. Possible relevant pathological findings are reduced urinary bladder capacity, multiple small diverticula (see fig. Christmas tree bladder), residual urine, and signs of subvesical obstruction or vesicoureteral reflux.
Urodynamics:
The exact type of NLUTD cannot be reliably predicted from the clinical symptoms only, but not all patients require urodynamic studies. It is necessary for patients with unsuccessful conservative therapy, for neurological diseases with a risk of DSD and high evacuation pressures, and before invasive therapy. See the section "urodynamic studies" for technical details and typical findings in neurogenic lower urinary tract dysfunction.
Differential Diagnosis
Overactive bladder, dysfunctional voiding, BPH, bladder cancer, bladder stones, and urinary tract infection.
Treatment of NLUTD — Improvement of Bladder Storage Function
Behavioral therapy:
Indications for behavioral therapy are urge symptoms or mild urge incontinence. Treatment includes reducing the fluid intake to a reasonable amount, micturition at set time intervals, and pelvic floor exercises with electrical stimulation, often combined with drug therapy.
Anticholinergic therapy:
Anticholinergics act as antagonists on muscarinic receptors; this leads to a relaxation of the smooth muscles. The force of normal or autonomous detrusor contractions is reduced, and the functional urinary bladder capacity is increased.
Regular micturition is necessary to obtain significant symptomatic improvement. The aim is to urinate before the bladder volume is reached, which induces an autonomous detrusor contraction. The high rate of bothersome side effects often leads to termination of drug therapy. The following substances are often used to treat NLUTD; for a detailed description of pharmacology, see the section "anticholinergics":
- Trospium: 15 mg 1-1-1.
- Oxybutynin: 2.5 mg 1-0-1 to 5mg 1-1-1-1.
- Tolterodine: 1 mg 1-0-1 to 2 mg 1-0-1. Extended-release 2–4 mg 1-0-0.
- Fesoterodine: 4–8 mg 1-0-0.
- Propiverine: 15 mg 1-0-1, or increase to 1-1-1.
- Darifenacin: 7.5–15 mg 1-0-0.
- Solifenacin: 5–10 mg 1-0-0.
Adrenergic β3 Receptor Agonists:
Adrenergic β3 receptors occur in the urinary bladder and mediate (hardly) any cardiovascular effects. Selective β3 receptor agonists such as mirabegron and vibegron proved effective for the therapy of OAB in clinical studies with few side effects. Mirabegron is approved for the treatment of neurogenic detrusor overactivity in children. The use in adults for neurogenic detrusor overactivity is Off-Label but recommended in many guidelines as an alternative to antimuscarinics. The dosage of mirabegron is 50 mg 1-0-0 p.o. A dose reduction to 25 mg 1-0-0 p.o. is recommended for patients with severe renal insufficiency or moderate hepatic insufficiency. The dosage of vibegron is 75 mg p.o. once daily.
Botulinum Toxin:
Transurethral injections of botulinum toxin A into the detrusor reduce detrusor overactivity lasting several months with increased functional urinary bladder capacity. Botulinum toxin A is indicated in case of failure or intolerance of medical therapy (Cruz et al., 2011); see above. The dosage (e.g., 100–00 units of Botox) must be titrated according to the effects and side effects, especially if the patient micturates spontaneously. If the dosage is too high, residual urine and urinary retention might necessitate intermittent self-catheterization (CIC). See also the section Pharmacology of botulinum toxin A.
Sacral neuromodulation:
The electrical stimulation of the S3 dorsal root leads to an inhibition of the micturition reflex and, thus, to a reduction of detrusor overactivity and urge incontinence.
Indication:
Sacral neuromodulation is indicated for neurogenic detrusor overactivity, which is not sufficiently responsive to medication. Before the subcutaneous pulse generator is implanted, the potential therapeutic success is tested with the help of a percutaneous test stimulation lasting several days.
Outcome:
50% of the patients receive the pulse generator after percutaneous testing. After four years, 40% were cured and 20% reported improved symptoms.
Percutaneous neuromodulation:
Weekly tibial nerve stimulation via a percutaneous needle at the ankle leads to a symptom improvement of 60%.
Bladder denervation:
Various techniques of bladder denervation exist on the spinal or peripheral level. Since better treatment options are available, bladder denervations techniques are rarely used, e.g., for severe autonomic dysreflexia. Side effects on other organ systems (rectum, sensitivity) and the regeneration of the nervous system (neuroplasty) may lead to even worse outcomes.
Bladder augmentation:
Bladder augmentation is used to increase the bladder capacity. Good results can be expected if the bladder sphincter control is maintained. Residual urine may develop postoperatively, and the patient should be able to perform self-catheterization (CIC). Postoperative urinary retention with low filling pressures and the need for CIC is, however, significantly better for the patient than detrusor hyperreflexia with high micturition pressures and the risk of kidney damage. Numerous bladder augmentation techniques exist:
- Bladder augmentation with preservation of the urothelium (detrusor myotomy)
- Bladder augmentation using the digestive tract (stomach, ileum or colon)
- Bladder augmentation with ureter if a severely dilated ureter of a nonfunctioning kidney is available.
Treatment of NLUTD — Improvement of Continence
Drug therapy:
Duloxetine is a serotonin and norepinephrine reuptake inhibitor (SSRI) on the spinal level and reinforces the strength of the sphincter contraction. Duloxetine has been approved for the treatment of mild stress urinary incontinence in Europe for women. Duloxetine failed the US approval for stress urinary incontinence amidst concerns over liver toxicity and suicidal events.
Artificial sphincter:
An artificial sphincter is an option to treat sphincter insufficiency, see also section surgical treatment of stress incontinence. The artificial sphincter is not an option for patients with uncontrolled detrusor overactivity or missing manual dexterity to operate the sphincter. The sphincter cuff should be placed around the bladder neck in patients requiring CIC due to insufficient bladder emptying.
Bladder neck reconstruction:
Bladder neck reconstruction is an option to treat childhood sphincter insufficiency due to, e.g., spina bifida. The published case numbers are low and of variable clinical success. The bladder neck reconstruction creates a valve-like continence mechanism, e.g., Young-Dees-Leadbetter bladder neck repair. In patients with neurogenic bladder dysfunction, augmentation and CIC are also necessary, depending on the bladder function.
Treatment of NLUTD — Emptying of the Bladder
Manual compression (Credé) or Valsalva maneuver:
It may cause high pressure in the bladder (up to 50 cm H2O) with the risk of upper urinary tract deterioration.
Manual triggering of the micturition reflex:
The micturition reflex is usually triggered by tapping the suprapubic bladder region but is often inadequate.
Parasympathomimetics:
Parasympathomimetics increase detrusor contraction by stimulating M-receptors on the urinary bladder. They have been used to treat postoperative urinary retention and neurogenic detrusor insufficiency. Parasympathomimetics have limited practical significance, due to potential severe cardiovascular side effects and questionable efficacy. Dosage of Bethanechol: 10 mg 1-1-1 up to 50 mg 1-1-1-1 p.o.; gradually increase the dosage according to results.
Intermittent self-catheterization:
Intermittent self-catheterization (ISC) or clean intermittent catheterization (CIC) effectively treats insufficient bladder emptying due to NLUTD or after bladder augmentation. In patients with CIC, detrusor overactivity can be treated with high doses of botulinum toxin or bladder augmentation. The long-term safety of the method was popularized by Lapides using clean (non-sterile reuse) catheters (Lapides et al., 1972). Still, sterile hydrophilic-coated single-use catheters may offer significant advantages. Complications include urethral injury (via falsa), urethral stricture, and urinary tract infections; see section "catheters".
Indwelling catheterization:
Urethral catheterization in women and suprapubic catheterization in men. Indwelling catheterization has a very high complication rate in the long term: urinary tract infections, stone formation, hematuria, pain, chronic kidney failure, and the development of squamous cell carcinoma. See also the section "catheters". Depending on the age and underlying diseases, alternatives (CIC, condom catheter, urinary diversion) are preferable.
Sacral neuromodulation:
S3 sacral nerve stimulation can also improve detrusor contraction in selected patients. Neuromodulation prevents autonomous contractions during the storage phase; pausing neuromodulation leads to a more effective voiding.
Further treatment options:
- bladder reduction surgery (controversial)
- bladder myoplasty (complex and low number of cases)
- transurethral electrical bladder stimulation (TEBS) to train the micturition reflex (controversial)
Treatment of NLUTD — Decreasing Outlet Resistance
α-Adrenoreceptor antagonists:
Alpha blockers are indicated for smooth muscle sphincter or bladder neck dyssynergia; for pharmacology, see the section section alpha blockers.
Baclofen:
Baclofen activates GABAB receptors and thereby inhibits monosynaptic or polysynaptic activation of the motor neurons. Indications for baclofen are the relief of motor spasticity and detrusor sphincter dyssynergia. Dosage: starting with 5 mg 1-1-1, increase dosage by 5 mg to approx. 30–75 mg/day.
Botulinum toxin:
Transurethral injection into the sphincter to treat detrusor sphincter dyssynergia may be combined with injections into the detrusor to treat detrusor overactivity. For pharmacology, dosage, and side effects, see section botulinum toxin.
Transurethral incision of the prostate (TUIP):
A transurethral incision of the prostate (TUIP) is a deep incision at 5 and 7 o'clock from the bladder neck to the seminal colliculus. It is a treatment option for dysfunctional micturition or smooth muscle sphincter or bladder neck dyssynergia in men.
Transurethral sphincterotomy:
The deep incision of the sphincter at 12 o'clock creates stress incontinence and lowers the detrusor leak point pressure (DLPP). After surgery, the patient is provided with a condom catheter. For women, sphincterotomy is a poor therapeutic option, since effective drainage systems cannot be applied.
The incision must extend from the verumontanum to the bulbomembranous urethra. The DLPP after sphincterotomy should be less than 40 cm H2O. A relatively high failure rate in the long-term course of up to 50% results from the regeneration of the sphincter or due to urethral stricture. Alternatively, a sphincter stent can be used. Close monitoring of the upper urinary tract is necessary.
Treatment of NLUTD — Permanent Urinary Diversion
Urinary diversion should be considered after failure of the above-mentioned treatment options (recurrent urinary tract infections, deterioration of the upper urinary tract, need for indwelling catheter). Treatment options depend on patient preferences, manual dexterity, renal function, and underlying diseases: ileovesicostomy, Mitrofanoff appendicovesicostomy, ileal conduit, continent heterotope urinary diversion (e.g., Mainz-Pouch I).
| Etiology of NLUTD | Index | Enuresis |
Index: 1–9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
References
Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.;
Rosier, P.; Ulmsten, U.; van Kerrebroeck, P.; Victor, A.; Wein, A. & of
the International Continence Society, S. S.
The standardisation of
terminology of lower urinary tract function: report from the
Standardisation Sub-committee of the International Continence Society.
Neurourol
Urodyn, 2002, 21, 167–178.
A. W. Partin, C. A. Peters, L. R. Kavoussi, R. R. Dmochowski, and A. J. Wein, Campbell-Walsh-Wein Urology, 12th ed. ISBN-13: 978-1455775675: Elsevier, 2020.
Chapple u.a. 2005 CHAPPLE, C. ; KHULLAR, V. ;
GABRIEL, Z. ; DOOLEY, J. A.:
The effects of antimuscarinic treatments in overactive bladder: a
systematic review and meta-analysis.
In: Eur Urol
48 (2005), Nr. 1, S. 5–26
van Kerrebroeck 1998 KERREBROECK, P. E. V. van:
Neurogenic Bladder Dysfunction.
In: Eur Urol
Curric Urol 4.2 (1998), S. 1–9
Wein und Rackley 2006 WEIN, A. J. ; RACKLEY,
R. R.:
Overactive bladder: a better understanding of pathophysiology,
diagnosis and management.
In: J Urol
175 (2006), Nr. 3 Pt 2, S. S5–10
 Deutsche Version: Neurogene Funktionsstörungen des unteren Harntrakts
Deutsche Version: Neurogene Funktionsstörungen des unteren Harntrakts
Urology-Textbook.com – Choose the Ad-Free, Professional Resource
This website is designed for physicians and medical professionals. It presents diseases of the genital organs through detailed text and images. Some content may not be suitable for children or sensitive readers. Many illustrations are available exclusively to Steady members. Are you a physician and interested in supporting this project? Join Steady to unlock full access to all images and enjoy an ad-free experience. Try it free for 7 days—no obligation.
New release: The first edition of the Urology Textbook as an e-book—ideal for offline reading and quick reference. With over 1300 pages and hundreds of illustrations, it’s the perfect companion for residents and medical students. After your 7-day trial has ended, you will receive a download link for your exclusive e-book.