You are here: Urology Textbook > Bladder > Bladder cancer Treatment
Bladder Cancer: Surgical Treatment (TURB and Cystectomy)
Review Literature: EAU guidelines superficial bladder cancer. EAU guidelines of muscle-invasive and metastatic bladder cancer. German S3 guidelines bladder carcinoma Harnblasenkarzinom.
- Bladder carcinoma: Definition, Epidemiology and Etiology
- Bladder carcinoma: Pathology and TNM tumor stages
- Bladder carcinoma: Symptoms and Diagnosis
- Bladder carcinoma: Surgical Treatment
- Bladder carcinoma: Chemotherapy and Immunotherapy of Metastases
Overview of Treatment Options
See the flowchart below for the treatment options of bladder cancer.
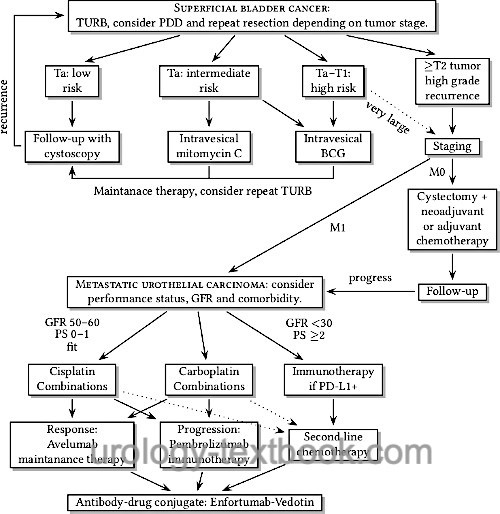 |
Treatment Options of Superficial Bladder Cancer:
TURB with complete resection of the tumor, single shot mitomycin C after TURB. Repeat resection of the scar after six weeks is recommended for any tumor but small unilocular Ta low-grade. Regular follow-up depends on the risk classification according to table table simple risk stratification, EORTC risk classification 1 and tab. EORTC risk classification 2.
Endoscopically unmanageable tumor:
Radical cystectomy.
Adjuvant intravesical therapy:
The adjuvant intravesical therapy is possible with mitomycin C or BCG, depending on the risk profile according to the table simple risk stratification. Fluorescence cystoscopy, repeat scar resection, and quadrant biopsy are done after the first treatment cycle.
Scar resection with a negative histology:
Intravesical maintenance therapy.
Scar resection with a low-grade recurrence:
Further intravesical chemotherapy and repeat TURB.
Scar resection with a high-grade recurrence:
Radical cystectomy.
Treatment Options of muscle-invasive bladder cancer (T2–4 M0):
Radical cystectomy. Consider neoadjuvant chemotherapy for advanced bladder cancer.
After cystectomy with R0, N0, M0:
Regular cancer follow-up.
After cystectomy with R1, N+ or ≥T3b:
Consider adjuvant chemotherapy or immunotherapy. Regular cancer follow-up.
Urothelial carcinoma of the prostate:
Radical cystectomy; orthotopic urinary diversion is not possible.
Alternatives to cystectomy:
Alternatives to cystectomy should be considered in patients with a high surgical risk.
≥T2 bladder carcinoma at the bladder dome:
Partial cystectomy with pelvic lymph node dissection.
Repeat radical TURB:
If a R0 resection is achieved, continue with intravesical therapy and close follow-up with repeat biopsies.
Radiochemotherapy:
Radical TURB with adjuvant radiochemotherapy.
Bladder carcinoma with distant metastases:
First line therapy with enfortumab vedotin + pembrolizumab (EV+P), second line therapy with platinum-based chemotherapy, immune-checkpoint inhibitors or erdafitinib.
Transurethral Resection of the Bladder (TURB)
Transurethral resection of the bladder (TURB) is the initial form of therapy for all bladder tumors. The depth of infiltration and histological differentiation can be diagnosed relatively accurately, and further therapeutic steps are planned depending on these results (see above). It is crucial to include bladder muscle in the resection specimen for accurate histopathological staging, there is a significant risk of understaging due to insufficient resection depth. The aim of resection is the complete removal of the tumor manifestation, for this purpose the tumor is either resected in fractions (standard method) or dissected en bloc with tumor base from the bladder wall.
Repeat transurethral resection:
Patients with small, unilocular, noninvasive, and well-differentiated bladder carcinomas (Ta low-grade) are adequately treated with a single resection and can be followed with cystoscopies. A repeat resection of the scars within six weeks is indicated for any other superficial bladder carcinoma with higher risk (large, multilocular, T1, or high-grade tumors). For TaG3 or T1 tumors, the risk of residual tumor in the specimen of the repeat resection is up to 30–50%, and the repeat resection leads to a significant improvement in the recurrence rate.
En-bloc resection:
En-bloc resection is done with a monopolar hook, the resection loop, or using laser techniques. En-bloc resection offers the advantage of reduced tumor cell seeding and improves pathologic examination; comparative studies are lacking.
Fluorescence Cystoscopy:
See section "diagnosis of bladder cancer" for images and technique of fluorescence cystoscopy. Indications: high-grade tumor cells in urine cytology, in all patients with a history of multifocal or high-grade tumors.
Perioperative intravesical chemotherapy:
Early single-shot intravesical instillation of mitomycin C after TURB reduces the recurrence rate by 40% (Sylvester et al., 2004). Dosage: 40 mg intravesical mitomycin C, exposure time 1–2 hours (no irrigation or gravity drainage). Contraindications: permanent postoperative bleeding, bladder perforation.
Intravesical Therapy
Intravesical therapy can safely reduce the risk of recurrence and progression of superficial bladder carcinomas. A simple risk assignment of recurrence and progression is possible with table simple risk stratification. More precisely, the probability of recurrence and progression can be determined using the EORTC risk classification (table EORTC risk classification 1 and table EORTC risk classification 2). Intravesical therapy is not recommended if the risk of recurrence and progression is low. For intermediate and high risk of recurrence or intermediate risk of progression, mitomycin C or BCG may be chosen as intravesical therapy. Intravesical therapy with BCG is more effective but has significantly more adverse effects. If the risk of progression is high, BCG should be chosen as the more effective agent, or, in young patients, early cystectomy is a reasonable alternative to intravesical therapy.
| Low-risk | First diagnosis of Ta low-grade tumor <3 cm |
| Intermediate risk | Low or high-risk criteria do not apply. |
| High-risk | T1 tumors, high-grade histology, CIS, multilocular recurrent low-grade tumors, tumor size > 3 cm. |
| Risk factor | Recurrence score | Progression score |
| Number of tumors | ||
| 1 | 0 | 0 |
| 2–7 | 3 | 3 |
| ≥8 | 6 | 3 |
| Tumor size | ||
| <3 cm | 0 | 0 |
| ≥3 cm | 3 | 3 |
| Tumor recurrence rate | ||
| First manifestation | 0 | 0 |
| ≤ 1 recurrence/year | 2 | 2 |
| >1 recurrence/year | 4 | 2 |
| T stage | ||
| Ta | 0 | 0 |
| T1 | 1 | 4 |
| Concurrent CIS | ||
| without CIS | 0 | 0 |
| with CIS | 1 | 6 |
| Grading | ||
| G1 | 0 | 0 |
| G2 | 1 | 0 |
| G3 | 2 | 5 |
| Recurrence risk | 1 year [%] | 5 years [%] |
| Low (0 points) | 15 | 31 |
| Intermediate (1–4 points) | 24 | 46 |
| Intermediate (5–9 points) | 38 | 62 |
| High (10–17 points) | 61 | 78 |
| Progression risk | 1 year [%] | 5 years [%] |
| Low (0 points) | 0,2 | 0,8 |
| Intermediate (2–6 points) | 1 | 6 |
| Intermediate (7–13 points) | 5 | 17 |
| High (14–23 points) | 17 | 45 |
Intravesical Therapy with Bacillus Calmette-Guerin (BCG):
The attenuated strain of Mycobacterium bovis leads to a local inflammatory reaction, which has an anticarcinogenic effect.
Indications for BCG:
Intravesical immunotherapy to lower recurrence and progression of intermediate- and high-risk superficial bladder carcinoma, see table EORTC risk classification 1 and table EORTC risk classification 2. BCG immunotherapy is more effective than mitomycin~C instillation; disadvantageous is the high rate of adverse reactions, see section pharmacology of BCG.
Dosage of BCG:
2×108 to 3×109 bacteria in 50 ml NaCl as intravesical instillation once weekly over six weeks. The recommended exposure time is two hours. After the six-week treatment cycle and six weeks off, a repeat resection with a quadrant biopsy should be performed. If intravesical chemotherapy is successful, maintenance therapy is reasonable: three instillations at weekly intervals at months 3, 6, 12, 18, 24, 30, and 36. In the case of high-grade recurrence, cystectomy should be sought. Alternatively, a second cycle of BCG may be attempted if the surgical risk is high.
Side effects:
Intravesical administration of BCG often leads to local and sometimes systemic side effects, which necessitate pausing or discontinuation of therapy. Please see the section "pharmacology of BCG".
Treatment results:
In superficial high-grade cancer, a durable remission is achievable in approximately 50–70%. BCG reduces the risk of recurrence by approximately 50% compared to TURB alone.
Intravesical Therapy with Mitomycin C:
Intravesical chemotherapy with mitomycin C is better tolerated than intravesical BCG; it is a treatment option instead of BCG for tumors with intermediate risk of recurrence or progression, see table EORTC risk classification 1 and table EORTC risk classification 2. The efficacy of mitomycin C is lower than BCG and should not be recommended in patients with high clinical risk.
Dosage of Mitomycin C:
20–40 mg of mitomycin C intravesically per week (6 instillations), then monthly administrations (11 instillations). See section mitomycin C for details on pharmacology and side effects.
Intravesical Therapy with Epirubicin or Doxorubicin:
Epirubicin or doxorubicin are alternatives to BCG or mitomycin C for intravesical therapy in superficial high-risk bladder carcinoma. Mechanism of action of the anthracyclines: interaction with DNA and RNA leads to impairment of synthesis and strand breaks.
Radical Cystectomy for Bladder Cancer Treatment
Indications for cystectomy:
- Bladder carcinoma with tumor stage greater than T1.
- Superficial high-grade bladder carcinoma with a high risk of progression in young patients ("early cystectomy"), see table EORTC risk classification 1 and table EORTC risk classification 2.
- Recurrence of a superficial high-grade tumor.
- Endoscopically uncontrollable superficial carcinoma.
- Contracted bladder after intravesical chemotherapy.
- Palliative cystectomy for severe local symptoms in patients with metastatic bladder carcinoma.
Surgical technique of cystectomy and urinary diversion:
The bladder is removed with a safe margin, and a pelvic lymphadenectomy is performed. In men, the specimen includes the bladder, prostate, seminal vesicles, distal ureters, and both ampulla vas deferens. In women, the specimen includes the bladder, distal ureters, segments of the anterior vaginal wall, uterus, and depending on age, the fallopian tubes and ovaries. If an orthotopic neobladder is not considered, the female urethra is also resected. See section cystectomy and urinary diversion for details.
Lymphadenectomy:
The dissection borders for standard lymphadenectomy are laterally the genitofemoral nerve, caudally the superior ramus of the os pubis, medially the umbilical ligament and the urinary bladder, dorsally the obturator nerve and the pelvic diaphragm, cranially the ureter and the bifurcation of the common iliac artery. In two prospective randomized studies, extended lymphadenectomy did not improve survival (Gschwend et al., 2019a).
Surgical modifications of cystectomy:
To avoid sexual side effects and improve urinary incontinence, technical modifications have been published in both women (Koie et al., 2010) and in men (Mertens et al., 2014). Preserving the vaginal anterior wall and uterus in women improves sexual function and continence after surgery. In men, preservation of the prostatic capsule protects the urinary sphincter and cavernous nerves with improved continence and erectile function. Careful selection of patients depending on intravesical tumor growth is elementary for oncological success. Preoperative targeted biopsies of the trigonum and the prostatic urethra should be cancer-free.
Oncological results after cystectomy:
10-year recurrence-free survival 80% (organ-confined, pN0), 61% (non-organ-confined, pN0), 45% (infiltration of adjacent organs, pN0), 34% for patient with pN1. The median time to recurrence is 12 months (4 months to 10 years) (Stein et al., 2001).
The median survival time for solitary lymph node metastasis after radical cystectomy is 30 months, and the five-year survival rate is 33%. Multiple lymph node metastases are significantly worse.
Neoadjuvant or Adjuvant Chemotherapy:
Several studies demonstrate prolonged survival with neoadjuvant and adjuvant chemotherapy; meta-analyses found an improved five-year survival rate of 5–6% (Burdett et al., 2022). Indications are extravesical tumor growth or lymph node metastases diagnosed preoperatively on imaging or after surgery with the pathological examination. Some authors recommend (neo)adjuvant therapy for every patient with muscle-invasive carcinoma.
Dosage:
2–4 cycles of MVAC or gemcitabine/cisplatin are administered before surgery, and further postoperative chemotherapy is decided upon on histology and response. The dosage for adjuvant chemotherapy is four cycles of MVAC or gemcitabine/cisplatin.
Adjuvant immunotherapy:
In patients at high risk of recurrence (pT3–4 or pN+), nivolumab improved disease-free survival after cystectomy: 21 months (nivolumab) vs. 11 months (placebo) (Bajorin et al., 2021). In Europe, approval was restricted to patients with tumor-cell PD-L1 expression ≥1%.
Adjuvant immunotherapy with atezolizumab failed to show improvement in progression-free survival (Bellmunt et al., 2021), and other checkpoint inhibitors are in clinical trials.
Neoadjuvant radiotherapy:
Neoadjuvant radiotherapy is not recommended before radical cystectomy, although individual studies have demonstrated a positive effect against local recurrence. Survival is determined by distant metastases which are not affected by neoadjuvant radiotherapy.
Partial Cystectomy
Partial cystectomy with pelvic lymphadenectomy is a therapeutic alternative to radical cystectomy for localized bladder carcinoma with muscle invasion, especially when localized at the bladder roof or an adequate distance from the ureteral orifices. Furthermore, urachal carcinoma and bladder diverticula carcinomas are well suited for partial cystectomy; this also applies to large superficial diverticula carcinoma. The disadvantage of partial cystectomy is the risk of intravesical recurrence and, thus, the need for regular cystoscopy and biopsies. Two-stage radical cystectomy for tumor progression is necessary in up to 25%. The following conditions are recommended to achieve oncologic outcome comparable to radical treatment (Capitanio et al., 2009):
- Solitary urinary bladder cancer.
- Exclusion of CIS by quadrant biopsy.
- Sufficient urinary bladder capacity allowing resection with a safety margin of 1–2 cm.
Bladder Preservation with Radiochemotherapy
Bladder preservation is possible with a combined therapy regimen: radical TURB is followed by radiotherapy and cisplatin-containing chemotherapy. Comparable results are reported: five-year survival of 48–63%, and in 36–43%, long-term survival with bladder preservation is reported.
Disadvantages include the high rate of side effects, the need for costly interdisciplinary treatment, and the high recurrence rate of superficial tumors with persistent need for cystoscopy and TURB. A clear advantage in quality of life seems more than questionable given the good functional results of the neobladder.
| Bladder cancer: diagnosis | Index | Bladder cancer chemotherapy |
Index: 1–9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
References
Abol-Enein, H.
Infection: is it a cause of bladder
cancer?
Scand J Urol Nephrol Suppl, 2008, 79-84.
Amin und Young 1997 AMIN, M. B. ; YOUNG, R. H.:
Primary carcinomas of the urethra.
In: Semin Diagn Pathol
14 (1997), Nr. 2, S. 147–60
Babjuk, M.; Burger, M.; Compérat, E.; Gonter, P.;
Mostafid, A.; Palou, J.; van Rhijn, B.; Rouprêt, M.; Shariata, S.;
Sylvester, R. & Zigeuner, R.
Non-muscle-invasive Bladder CancerEAU
Guidelines, 2020 https://uroweb.org/guidelines/non-muscle-invasive-bladder-cancer/
Brinkman, M. & Zeegers, M. P.
Nutrition, total
fluid and bladder cancer.
Scand J Urol Nephrol Suppl, 2008,
25-36.
Cohn, J. A.; Vekhter, B.; Lyttle, C.; Steinberg,
G. D. & Large, M. C.
Sex disparities in diagnosis of bladder cancer
after initial presentation with hematuria: a nationwide claims-based
investigation.
Cancer, 2014, 120, 555-561
DGU; DKG; DKG & Leitlinienprogramm Onkologie S3-Leitlinie (Langfassung): Früherkennung, Diagnose, Therapie und Nachsorge des Harnblasenkarzinoms. https://www.leitlinienprogramm-onkologie.de/leitlinien/harnblasenkarzinom/
J. E. Gschwend et al., “Extended Versus Limited Lymph Node Dissection in Bladder Cancer Patients Undergoing Radical Cystectomy: Survival Results from a Prospective, Randomized Trial.,” Eur Urol, vol. 75, no. 4, pp. 604–611, 2019, doi: 10.1016/j.eururo.2018.09.047.
Helpap und Kollermann 2000 HELPAP, B. ;
KOLLERMANN, J.:
[Revisions in the WHO histological classification of urothelial
bladder tumors and flat urothelial lesions].
In: Pathologe
21 (2000), Nr. 3, S. 211–7
IARC (2004) Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 83. Tobacco Smoke and Involuntary Smoking. World Health Organization.
Kalble 2001 KALBLE, T.:
[Etiopathology, risk factors, environmental influences and
epidemiology of bladder cancer].
In: Urologe A
40 (2001), Nr. 6, S. 447–50
Kataja und Pavlidis 2005 KATAJA, V. V. ;
PAVLIDIS, N.:
ESMO Minimum Clinical Recommendations for diagnosis, treatment and
follow-up of invasive bladder cancer.
In: Ann Oncol
16 Suppl 1 (2005), S. i43–4
Krieg und Hoffman 1999 KRIEG, R. ; HOFFMAN, R.:
Current management of unusual genitourinary cancers. Part 2: Urethral
cancer.
In: Oncology (Williston Park)
13 (1999), Nr. 11, S. 1511–7, 1520; discussion 1523–4
Lammers, R. J. M.; Witjes, W. P. J.; Hendricksen, K.;
Caris, C. T. M.; Janzing-Pastors, M. H. C. & Witjes, J. A.
Smoking
status is a risk factor for recurrence after transurethral resection of
non-muscle-invasive bladder cancer.
Eur Urol, 2011,
60, 713-720
Lampel und Thuroff 1998a LAMPEL, A. ;
THUROFF, J. W.:
[Bladder carcinoma 1: Radical cystectomy, neoadjuvant and adjuvant
therapy modalities].
In: Urologe A
37 (1998), Nr. 1, S. 93–101
Lampel und Thuroff 1998b LAMPEL, A. ;
THUROFF, J. W.:
[Bladder carcinoma. 2: Urinary diversion].
In: Urologe A
37 (1998), Nr. 2, S. W207–20
Leppert u.a. 2006 LEPPERT, J. T. ; SHVARTS,
O. ; KAWAOKA, K. ; LIEBERMAN, R. ; BELLDEGRUN,
A. S. ; PANTUCK, A. J.:
Prevention of bladder cancer: a review.
In: Eur Urol
49 (2006), Nr. 2, S. 226–34
Liu, S.; Yang, T.; Na, R.; Hu, M.; Zhang, L.; Fu,
Y.; Jiang, H. & Ding, Q.
The impact of female gender on bladder
cancer-specific death risk after radical cystectomy: a meta-analysis of
27,912 patients.
International urology and nephrology, 2015,
47, 951-958
Michaud u.a. 1999 MICHAUD, D. S. ; SPIEGELMAN,
D. ; CLINTON, S. K. ; RIMM, E. B. ; CURHAN,
G. C. ; WILLETT, W. C. ; GIOVANNUCCI, E. L.:
Fluid intake and the risk of bladder cancer in men.
In: N Engl J Med
340 (1999), Nr. 18, S. 1390–7
Plna und Hemminki 2001 PLNA, K. ; HEMMINKI, K.:
Familial bladder cancer in the National Swedish Family Cancer
Database.
In: J Urol
166 (2001), Nr. 6, S. 2129–33
Rajan u.a. 1993 RAJAN, N. ; TUCCI, P. ;
MALLOUH, C. ; CHOUDHURY, M.:
Carcinoma in female urethral diverticulum: case reports and review of
management.
In: J Urol
150 (1993), Nr. 6, S. 1911–4
Robert-Koch-Institut (2015) Krebs in Deutschland 2011/2012. www.krebsdaten.de
Stein u.a. 2001 STEIN, J. P. ; LIESKOVSKY,
G. ; COTE, R. ; GROSHEN, S. ; FENG, A. C. ;
BOYD, S. ; SKINNER, E. ; BOCHNER, B. ;
THANGATHURAI, D. ; MIKHAIL, M. ; RAGHAVAN, D. ;
SKINNER, D. G.:
Radical cystectomy in the treatment of invasive bladder cancer:
long-term results in 1054 patients.
In: J Clin Oncol
19 (2001), Nr. 3, S. 666–75
Weissbach 2001 WEISSBACH, L.:
[Palliation of urothelial carcinoma of the bladder].
In: Urologe A
40 (2001), Nr. 6, S. 475–9
Witjes, J.; Compérat, E.; Cowan, N.; Gakis, G.;
Hernánde, V.; Lebret, T.; Lorch, A.; van der Heijden, A. & Ribal, M.
Muscle-invasive
and Metastatic Bladder Cancer
EAU Guidelines, 2020 https://uroweb.org/guidelines/bladder-cancer-muscle-invasive-and-metastatic/
 Deutsche Version: Operative Therapie des Harnblasenkarzinoms: TURB und Zystektomie
Deutsche Version: Operative Therapie des Harnblasenkarzinoms: TURB und Zystektomie
Urology-Textbook.com – Choose the Ad-Free, Professional Resource
This website is designed for physicians and medical professionals. It presents diseases of the genital organs through detailed text and images. Some content may not be suitable for children or sensitive readers. Many illustrations are available exclusively to Steady members. Are you a physician and interested in supporting this project? Join Steady to unlock full access to all images and enjoy an ad-free experience. Try it free for 7 days—no obligation.
New release: The first edition of the Urology Textbook as an e-book—ideal for offline reading and quick reference. With over 1300 pages and hundreds of illustrations, it’s the perfect companion for residents and medical students. After your 7-day trial has ended, you will receive a download link for your exclusive e-book.