You are here: Urology Textbook > Drugs in Urology > Cephalosporins
Cephalosporins: Mechanism of Action, Adverse Effects and Contraindications
Four Generations of Cephalosporins
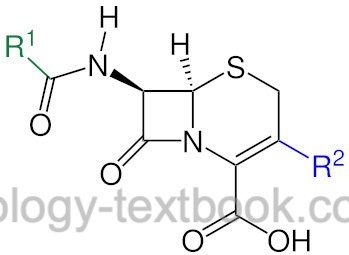
Cephalosporins are β-lactam antibiotics, which are grouped into four generations according to their antibiotic spectrum of activity:
- First generation: cefazolin
- Second generation: cefaclor, cefuroxime
- Third generation: cefotaxime, ceftriaxone, ceftazidime for parenteral application. Cefpodoxime and ceftibuten for oral formulations.
- Fourth generation: cefepime, ceftolozan (combined with tazobactam)
Mechanism of action of Cephalosporins
Cephalosporins are β-lactam antibiotics; they inhibit the peptidoglycan synthesis of the bacterial wall after binding to so-called penicillin-binding proteins (peptidoglycan synthetases) and inhibit the polymerization of the peptidoglycan and covalent cross-linking of the bacterial wall.
Antibiotic Spectrum of Different Cephalosporin Generations
The first generation has mainly gram-positive activity. The second and third generations have more gram-negative activity with decreased activity against gram-positive bacteria. The fourth generation of cephalosporins has a broad spectrum of activity.
Cephalosporins are generally ineffective against enterococci, Listeria, Legionella, Mycoplasma, and Chlamydia.
Adverse Effects of Cephalosporins
- Allergic reactions ranging from rash to anaphylaxis: 1–4%, the risk of cross-allergy to penicillin is approximately 10%.
- Gastrointestinal side effects: nausea and diarrhea are common, as are elevated liver enzymes; pseudomembranous enterocolitis is rare but possible.
- Rare but potentially serious adverse reactions include cerebral seizures, severe cutaneous skin reactions, anaphylaxis, interstitial nephritis, cholestatic jaundice (especially ceftriaxone), hemolytic anemia, and thrombocytopenia.
Drug Interactions with Cephalosporins
- Increased nephrotoxicity when combined with aminoglycosides or loop diuretics.
- Probenecid inhibits renal tubular excretion and increases serum concentration.
Contraindications
Allergy to cephalosporins. Caution is necessary in patients with a history of penicillin allergy.
Cefazolin
Cefazolin is a first-generation cephalosporin.
Antibiotic Spectrum of Cefazolin
Staphylococci (but not MRSA), streptococci, E. coli, Proteus, and Klebsiella.
Urologic Indications for Cefazolin
Staphylococcal infections (wound infections), urinary tract infections (if proven effective in the antibiogram), and perioperative antibiotic prophylaxis.
Pharmacokinetics of Cefazolin
Cefazolin is only available for parenteral administration. The elimination half-life is 1.5 h, with 90% renal elimination.
Dosage of Cefazolin
In adults, the dosage of cefazolin is 1 g 1-1-1 IV Children receive a daily dose of 60 mg/kgBW, divided into three doses. Dose reduction is necessary for chronic kidney disease.
Cefuroxime
Cefuroxime is a second-generation cephalosporin available in oral and parenteral formulations.
Antibiotic Spectrum of Cefuroxime
Staphylococci (except MRSA), streptococci, Haemophilus, E. coli, Proteus, Klebsiella, Salmonella, Shigella, Citrobacter.
Urologic Indications of Cefuroxime:
Perioperative antibiotic prophylaxis, urinary tract infections (if proven effective in the antibiogram).
Pharmacokinetics of Cefuroxime
Cefuroxime can be prescribed orally and intravenously. However, the oral bioavailability of cefuroxime is only 30–50%, and it is best taken after meals. Half-life 1 hour. Renal elimination 70–90%.
Dosage of Cefuroxime
For urinary tract infections in adults: cefuroxime 250–500 mg p.o. 1-0-1 or 1.5 g 1-1-1 IV Children receive 25 mg/kgBW 1-1-1 IV In severe infections, parenteral administration should be preferred due to poor oral bioavailability.
A dose reduction is necessary for renal insufficiency with a GFR below 20 ml/min: 0.75 g cefuroxime twice daily, and below 10 ml/min once daily, 0.75 g.
Cefotaxime
Cefotaxime is a third-generation cephalosporin available for parenteral use.
Antibiotic Spectrum of Cefotaxime
Effective against streptococci, gonococci, meningococci, Haemophilus, and staphylococci. Cefotaxime has a high β-lactamase resistance and is, therefore, effective against gram-negative bacteria (Enterobacteriaceae). Only weak efficacy against Pseudomonas. No efficacy against enterococci, mycoplasma, chlamydia, and MRSA.
Urologic Indications of Cefotaxime:
Severe complicated urinary tract infections and urosepsis.
Pharmacokinetics of Cefotaxime
Only parenteral administration of cefotaxime is possible. The half-life of cefotaxime is 1 hour, and significantly longer in patients with renal insufficiency. Hepatic metabolism to active metabolites, 80–90% renal elimination.
Dosage of Cefotaxime
The daily dosage of cefotaxime for adults is 3–6 g (children 50–100 mg/kgBW), divided into three individual doses, depending on the severity of the infection.
A dose reduction is necessary for patients with renal insufficiency. For a GFR below 50 ml/min, the daily dose (after the initial normal dose) is reduced to 50%. For a GFR below 10 ml/min, it is reduced to 25%.
Ceftriaxone
Ceftriaxone is a third-generation cephalosporin available for parenteral use.
Antibiotic Spectrum of Ceftriaxone
Comparable to cefotaxime, see above.
Urologic Indications for Ceftriaxone
Severe complicated urinary tract infections and urosepsis. Treatment of gonorrhea.
Pharmacokinetics of Ceftriaxone
Only parenteral administration of ceftriaxone is possible. High protein binding and good tissue penetration. The half-life of ceftriaxone is 6–9 hours. Modest hepatic metabolism. Biliary (40–50%) and renal (50–60%) elimination.
Dosage of Ceftriaxone
1–2 g ceftriaxone once daily IV for adults, depending on the severity of the infection. Children receive 50–80 mg/kgBW. For the treatment of gonorrhea, 500 mg ceftriaxone is administered IM or IV as a single dose. A dose reduction is not necessary for patients with renal insufficiency if liver function is not impaired.
Ceftibuten
Ceftibuten is an orally available third-generation cephalosporin.
Antibiotic Spectrum:
Compared to cefotaxime, it is highly effective against gram-negative bacteria but less effective against gram-positive bacteria.
Urological Indications of Ceftibuten
Outpatient treatment of urinary tract infections, also as oral step-down treatment after initial parenteral treatment of severe urinary tract infections.
Pharmacokinetics of Ceftibuten:
Good bioavailability when taken independently of meals, half-life 2.5–4 hours, low metabolism, and unchanged excretion via the urine.
Dosage of Ceftibuten:
For adults 400 mg 1-0-0, children 9 mg/kgBW 1-0-0. Halve the dosage for patients with a GFR of 50–30 ml/min. For a GFR of 29–5 ml/min, prescribe only a quarter of the normal dosage.
Cefixime
Cefixime is an orally available third-generation cephalosporin.
Antibiotic Spectrum of Cefixime:
Comparable to ceftibuten.
Urological Indications of Cefixime
Outpatient treatment of urinary tract infections, also as oral step-down treatment after initial parenteral treatment of severe urinary tract infections. Second-line treatment of gonorrhea.
Pharmacokinetics of Cefixime:
30–50% bioavailability independent of food intake, half-life 3–4 hours, modest metabolism, and unchanged excretion via the urine.
Dosage of Cefixime:
For adults 200 mg 1-0-1 or 400 mg 1-0-0, children 4 mg/kgBW 1-0-1 or 8 mg/kgBW 1-0-0. In patients with severe renal insufficiency (GFR <20 ml/min), the dose should be limited to 200 mg once daily.
Cefpodoxime
Cefpodoxime is an orally available third-generation cephalosporin.
Antibiotic Spectrum of Cefpodoxime:
Comparable to ceftibuten, it is slightly more effective against gram-positive bacteria (pneumococci or staphylococci).
Urological Indications of Cefpodoxime
Outpatient treatment of urinary tract infections, also as oral step-down treatment after initial parenteral treatment of severe urinary tract infections.
Pharmacokinetics of Cefpodoxime:
The prodrug (cefpodoxime proxetil) is hydrolyzed by the intestinal mucosa, with approximately 50% bioavailability when taken with food. Cefpodoxime has a half-life of 2.5 hours, low metabolism, and unchanged excretion via urine.
Dosage of Cefpodoxime:
For adults 100–200 mg 1-0-1 depending on the severity of the infection, children receive 4 mg/kgBW 1-0-1. In patients with renal insufficiency (GFR 40–10 ml/min), the dose should be limited to once daily. In severe renal insufficiency (GFR <10 ml/min), a single dose is administered every two days.
| Piperacillin/Tazobactam | Index | Carbapenems |
Index: 1–9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
References
Gok u.a. 2001 GOK, F. ; DUZOVA, A. ;
BASKIN, E. ; OZEN, S. ; BESBAS, N. ;
BAKKALOGLU, A.:
Comparative study of cefixime alone versus intramuscular ceftizoxime
followed by cefixime in the treatment of urinary tract infections in
children.
In: J Chemother
13 (2001), Nr. 3, S. 277–80
Ho u.a. 2001 HO, M. W. ; WANG, F. D. ;
FUNG, C. P. ; LIU, C. Y.:
Comparative study of ceftibuten and cefixime in the treatment of
complicated urinary tract infections.
In: J Microbiol Immunol Infect
34 (2001), Nr. 3, S. 185–9
Ovalle u.a. 2000 OVALLE, A. ; MARTINEZ,
M. A. ; WOLFF, M. ; CONA, E. ; VALDERRAMA, O. ;
VILLABLANCA, E. ; LOBOS, L.:
[Prospective, randomized, comparative study of the efficacy, safety
and cost of cefuroxime versus cephradine in acute pyelonephritis during pregnancy].
In: Rev Med Chil
128 (2000), Nr. 7, S. 749–57
H. R. Brodt, A. Hörauf, M. Kresken, W. Solbach, and T. Welte, Infektionstherapie: Antibiotika, Virostatika, Antimykotika, Antiparasitäre Wirkstoffe. Thieme, 2023.
Vilaichone u.a. 2001 VILAICHONE, A. ; WATANA,
D. ; CHAIWATANARAT, T.:
Oral ceftibuten switch therapy for acute pyelonephritis in children.
In: J Med Assoc Thai
84 Suppl 1 (2001), S. S61–7
 Deutsche Version: Cephalosporine
Deutsche Version: Cephalosporine
Urology-Textbook.com – Choose the Ad-Free, Professional Resource
This website is designed for physicians and medical professionals. It presents diseases of the genital organs through detailed text and images. Some content may not be suitable for children or sensitive readers. Many illustrations are available exclusively to Steady members. Are you a physician and interested in supporting this project? Join Steady to unlock full access to all images and enjoy an ad-free experience. Try it free for 7 days—no obligation.
New release: The first edition of the Urology Textbook as an e-book—ideal for offline reading and quick reference. With over 1300 pages and hundreds of illustrations, it’s the perfect companion for residents and medical students. After your 7-day trial has ended, you will receive a download link for your exclusive e-book.