You are here: Urology Textbook > Anatomy > Testes
Physiology of the Testis (Male Hormones): Testosterone and other Androgens
- Anatomy of the Testes: Gross appearance, vascular supply, innvervation, spermatic cord.
- Anatomy of the Testes: Histology, Spermatogenesis.
- Anatomy of the Testes: Male Hormones (Testosteron, LH, FSH, Inhibin).
References: (Benninghoff, 1993).
Sex differentiation
The Chromosomal Sex:
the normal human karyotype consists of 46 chromosomes, 22 pairs of autosomal chromosoms, and 2 sex chromosoms. Normal karyotypes for females contain two X chromosomes (46,XX), males have both an X and a Y chromosome (46,XY).
The chromosomal constellation induces the development of ovaries or testes from the undifferentiated gonads. The presence of a Y chromosome or the responsible region of the Y chromosome (testis-determining factor) leads to the development of a testicle. The responsible gene for testis determining factor is called SRY. SRY encodes a protein of 80 AS (high mobility group protein HMG), which binds at the DNA and causes the transcription of genes for the sex differentiation (WT-1, SF-1, SOX).
Without the presence of the testis-determining factor the undifferentiated gonad develops into an ovary. In the presence of two X chromosomes (or more) an X chromosome inactivation occurs: heterochromatin is formed, which is seen in the light microscope as Barr bodies. The inactivation and packaging of the excess X chromosome is encoded by the XIST gene.
Embryology of the Testes:
without the presence of a functioning testis with testosterone production a female genital develops in spite of the male sex chromosome (46,XY). This also applies to defects in the androgen receptor, lack of conversion of testosterone into dihydrotestosterone (DHT) or missing Anti-Müllerian hormone (AMH).
The Psychosexual Differentiation:
the earlier assumption, the psychological sexual identification is created through education and physical comparison, is false. Male patients (46,XY) with intersex disorder by enzyme defects often develop doubts whether their assigned female gender role. An embryonic exposition to androgens and (unknown) genes on the Y chromosome lead to early sexual imprinting.
Function and Control of the Male Hormones (Androgens)
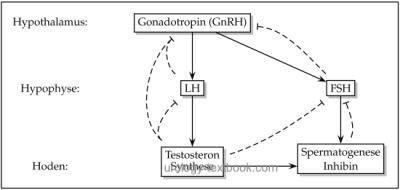
The hypothalamus-pituitary-gonadal hormone axis controls the release of sex hormones [fig. regulation of testosterone]. The androgens and their precursors play a crucial role in the development of sex phenotype, sexual maturation during puberty, control of the endocrine function of testis and spermatogenesis.
 |
Hypothalamus and Male Hormones:
The hypothalamus produces gonadotropin-releasing hormone (GnRH or LHRH) with the pulsatile releases every 90–120 min. GnRH, a peptide hormone of 10 amino acids, stimulates the pituitary to the release of LH and FSH. GnRH pulses are inhibited through sexual steroids, dopamine, FSH, LH, opioids, and probably also inhibin.
Pituitary Gland and Male Hormones:
GnRH enters the portal venous circulation of the pituitary gland and stimulates the release of peptide hormones luteinizing hormone (LH) and follicle-stimulating hormone (FSH). LH (28 kDa) and FSH (33 kDa) are glycoproteins, which consist of α-and β-chains. The α-chain is similar to the other pituitary hormones, the β-chain mediates the biological effects.
LH secretion is following the pulsatile GnRH secretion, FSH secretion is more complex due to the influence of inhibin and the longer half-life of FSH. LH induces testosterone synthesis in the testis, FSH is the key stimulus for spermatogenesis. Testosterone inhibits the release of LH, while Inhibin suppresses the release of FSH.
Testes and Male Hormones:
both basic functions of the testes, endocrine function (testosterone, inhibin ...) and the exocrine function (spermatogenesis), are controlled by the hypothalamic-pituitary-gonadal hormone axis.
Testosterone:
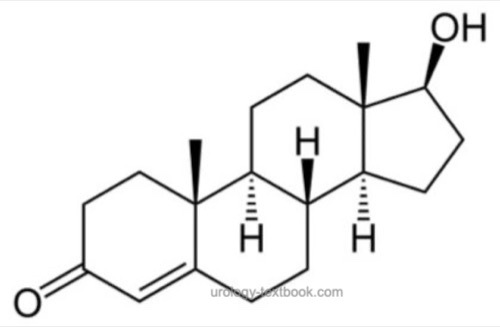
for the chemical structure of the steroid hormone see fig. chemical structure of testosterone. The biosynthesis of testosterone is presented in chapter Anatomy of the Adrenal Glands. Only 1–3% of total testosterone are unbound in plasma (free testosterone). The rest is bound to the sex hormone-binding globulin (SHBG) and to other proteins as albumin. Albumin-bound testosterone can also display biological activity. Therefor bioavailable testosterone is testosterone not bound to the SHBG (free testosterone + albumin-bound testosterone). The highest testosterone concentration in serum is measured in the morning, the reference range is between 12–35 nmol/l (3.5–10 ng/ml). In the evening, the concentration of testosterone is 40% less than in the morning.
Testosterone is often converted into dihydrotestosterone (DHT) in the target organ by the enzyme 5-α reductase. DHT has a higher binding affinity to the androgen receptor and a higher androgen activity in the target organ than testosterone.
The enzyme aromatase in peripheral tissues causes the conversion of testosterone to estradiol. Testosterone and estradiol inhibit the release of LH and FSH in the pituitary gland and GnRH in the hypothalamus (negative feedback mechanism).
caption>
 |
Androgen receptor:
nuclear receptor protein complex, which consists of a DNA-binding and ligand-binding region. The androgen receptor shows a close relationship to the glucocorticoid receptor. The binding of androgens to the androgen receptor leads to an increased transcription of specific genes that mediate the main effects of androgens. Early onset androgen effects can bei mediated through the androgen receptor by activating intracellular protein kinases and via the intracellular calcium concentration (Culig et al., 2003).
Inhibin:
32 kDa protein, which is secreted by Sertoli cells and specifically inhibits FSH release in the pituitary gland. Conversely, the release of inhibin is stimulated by FSH.
Activin:
protein hormone with homology to TGF-beta, stimulates the release of FSH in the pituitary gland. Activin receptors are also found in other organs, the precise physiological effect is unclear.
Spermatogenesis:
the presence of FSH in the testes is crucial in puberty for the growth of the seminiferous tubules. The binding of FSH to Sertoli cells results in the production of a number of mediators, which stimulate spermatogenesis: androgen-binding protein, transferrin, lactate, ceruloplasmin, clusterin, plasminogen activator, prostaglandins and growth factors.
Function of male hormones
Male hormones play a crucial role in the development of the sexual phenotyp, the sexual maturation during puberty, the endocrine function of testis and spermatogenesis (see above).
Besides of the sexual functions testosterone controls the growth of body hair and the beard, but not the scalp hair. It has an anabolic effect (muscle, bone, cartilage). Furthermore, testosterone controls the behavior (libido, drive, stamina and aggression) and affects the blood cells (erythropoietin and effects on blood stem cells) and liver.
| Testes Histology | Index | Abbreviations |
Index: 1–9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
References
Benninghoff 1993 BENNINGHOFF, A.: Makroskopische Anatomie, Embryologie und Histologie des Menschen.15. Auflage.
München; Wien; Baltimore : Urban und Schwarzenberg, 1993
Culig u.a. 2003 CULIG, Z. ; KLOCKER, H. ;
BARTSCH, G. ; STEINER, H. ; HOBISCH, A.:
Androgen receptors in prostate cancer.
In: J Urol
170 (2003), Nr. 4 Pt 1, S. 1363–9
 Deutsche Version: Androgene: Testosteron, Dihydrotestosteron. LH, FSH, Inhibin. GnRH.
Deutsche Version: Androgene: Testosteron, Dihydrotestosteron. LH, FSH, Inhibin. GnRH.
Urology-Textbook.com – Choose the Ad-Free, Professional Resource
This website is designed for physicians and medical professionals. It presents diseases of the genital organs through detailed text and images. Some content may not be suitable for children or sensitive readers. Many illustrations are available exclusively to Steady members. Are you a physician and interested in supporting this project? Join Steady to unlock full access to all images and enjoy an ad-free experience. Try it free for 7 days—no obligation.