You are here: Urology Textbook > Drugs in Urology > Dapoxetin
Adverse Effects, Contraindications and Dosage of Dapoxetine
Mechanism of Action of Dapoxetine
Dapoxetine is a selective serotonin reuptake inhibitor (SSRI) with a short duration of action. By inhibiting the serotonin transporter, it increases serotonin concentrations in the synaptic cleft, which delays the ejaculatory reflex and prolongs the intravaginal ejaculatory latency time (IELT).
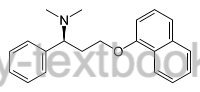 Structural formula of dapoxetine.
Structural formula of dapoxetine.
Indications for Dapoxetine
On-demand medication for the treatment of premature ejaculation in men 18 to 64 years of age (typically characterized by an IELT of less than two minutes, lack of control over ejaculation, marked distress, and interpersonal difficulties) in whom sexual counseling alone has not resulted in a satisfactory improvement.
Results of the Pivotal Trial
The largest randomized, placebo-controlled trial included 1,162 patients (Buvat 2009). Starting from a mean IELT of approximately one minute at baseline, mean IELT values after 24 weeks were 1.9 minutes with placebo, 3.2 minutes with dapoxetine 30 mg, and 3.5 minutes with dapoxetine 60 mg. Patients’ global assessment of treatment response after 24 weeks (placebo vs. 30 mg vs. 60 mg dapoxetine) showed no change or worsening in 68% vs. 42% vs. 28%, “a little better” or “better” in 28% vs. 48% vs. 60%, and “much better” in 4% vs. 10% vs. 12%, respectively.
Pharmacokinetics of Dapoxetine
Dapoxetine is rapidly absorbed from the gastrointestinal tract, with a maximum plasma concentration reached after 1 to 2 hours. Its bioavailability is approximately 42%, with considerable interindividual variability (15–76%), and food has only a minor effect on absorption. 99% plasma protein binding. Dapoxetine undergoes extensive metabolism in the liver and kidneys, primarily via the cytochrome P450 (CYP) isoenzymes CYP2D6 and CYP3A4 as well as monooxygenases, and its metabolites are eliminated predominantly via the kidneys. The initial elimination half-life is approximately 1.5 hours, whereas the terminal half-life of active and inactive metabolites ranges from 18 to 22 hours.
Adverse Effects of Dapoxetine
Nausea (2.2% of patients) and dizziness (1.2% of patients) are the most frequent adverse effects leading to discontinuation of dapoxetine treatment.
Central Nervous System (CNS):
Anxiety, restlessness, somnolence, headache, tremor, and dizziness occur commonly. Depressed mood, mood changes, disturbances in thinking, syncope, and other orthostatic reactions have been reported occasionally. Occurrence of depressive symptoms and suicidality is a general concern with SSRIs, there is no specific data for dapoxetine available.
Sensory Organs:
Visual disturbances, tinnitus, and dizziness have been reported occasionally.
Cardiovascular System:
Bradycardia, tachycardia, blood pressure fluctuations with hypotension or hypertension, as well as orthostatic hypotension and syncope may occur.
Gastrointestinal Tract:
Nausea occurs very frequently (in more than 10% of patients). Diarrhea, vomiting, abdominal pain, and flatulence occur frequently.
Drug Interactions
- Dapoxetine must not be coadministered with monoamine oxidase inhibitors (MAO inhibitors), selective serotonin reuptake inhibitors (SSRIs), serotonin–norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants, or other serotonergic agents (such as St. John’s wort, tramadol, triptans, linezolid, lithium, or thioridazine) because of the risk of serotonin syndrome.
- Dapoxetine must not be combined with potent CYP3A4 inhibitors such as ketoconazole, itraconazole, ritonavir, or similar agents, because these drugs can markedly increase dapoxetine plasma concentrations.
- Concomitant use of anticoagulants or antiplatelet agents may increase the risk of bleeding.
- Exercise caution when dapoxetine is used with other centrally acting depressants (such as alcohol, sedatives, or opioids) because of the potential for additive sedative effects.
Contraindications of Dapoxetine
- Moderate and severe hepatic impairment (Child–Pugh B and C).
- Severe renal impairment with an estimated glomerular filtration rate of less than 30 mL/min.
- Significant structural heart disease (such as heart failure New York Heart Association class II–IV, clinically relevant conduction disorders, significant coronary artery disease, or severe valvular heart disease).
- History of syncope.
- Mania, major depression, or ongoing psychiatric pharmacotherapy (see above).
- Dapoxetine is not approved for women, children, or adolescents.
Dosage of Dapoxetine
The recommended starting dose for on-demand use is 30 mg taken orally 1 to 3 hours before anticipated sexual activity. The maximum dosing frequency is once per day. If the response is inadequate and tolerability is good, the dose may be increased to 60 mg. If there is no meaningful improvement or if adverse effects are unacceptable, clinicians should discontinue treatment.
| Botulinum toxin A | Index | Desmopressin |
Index: 1–9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
References
Buvat, J.; Tesfaye, F.; Rothman, M.; Rivas, D. A. & Giuliano, F.
Dapoxetine for the Treatment of Premature Ejaculation: Results from a Randomized,
Double-Blind, Placebo-Controlled Phase 3 Trial in 22 Countries.
Eur Urol, 2009
 Deutsche Version: Nebenwirkungen und Kontraindikationen von Dapoxetin
Deutsche Version: Nebenwirkungen und Kontraindikationen von Dapoxetin
Urology-Textbook.com – Choose the Ad-Free, Professional Resource
This website is designed for physicians and medical professionals. It presents diseases of the genital organs through detailed text and images. Some content may not be suitable for children or sensitive readers. Many illustrations are available exclusively to Steady members. Are you a physician and interested in supporting this project? Join Steady to unlock full access to all images and enjoy an ad-free experience. Try it free for 7 days—no obligation.
New release: The first edition of the Urology Textbook as an e-book—ideal for offline reading and quick reference. With over 1300 pages and hundreds of illustrations, it’s the perfect companion for residents and medical students. After your 7-day trial has ended, you will receive a download link for your exclusive e-book.